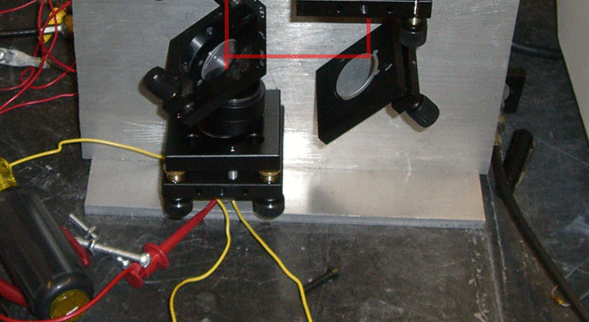
This experiment attempted to use an interferometer to detect bacteria. The basic idea behind an interferometer is to have a beam of collimated light which is split into two paths and then brought back together. Differences in path length cause interference patterns, or fringes. However, differences in path length need not only be caused by one beam of light traveling farther than the other. For example, if one beam goes through a glass plate, the index of refraction or n of the material will be greater than one. (n is greater than one in any medium besides a vacuum.) The greater n is, the slower the speed of light in that material.
c'=c/n
Since the speed of light is slower in the given material, having one beam travel through material with a higher index of refraction introduces a phase shift just as a difference in path length would have. Obviously, the phase shift is greater if n is greater and also greater if the material is thicker. Thus, the phase shift relative to the reference beam is (n' - n)/n * thickness of material.
There are other ways to create a phase shift as well. Here are some basic additional rules:
(This is because the light is acting like a wave on a string. If the n is greater behind the surface than in the surface the light is coming from, it is like the end of the string being fixed, causing a one-half wavelength phase shift. If the n is less behind the reflecting surface than in the medium where the wave is coming from, it is like the end of the string is free, so there is no phase shift.)
A Mach-Zender type inteferometer (which is the kind that we built) works by splitting the beam, passing one of the split beams through a sample (in this case, after focusing the beam to micron scale), then recombining the beams at a detector.
In theory, the ideal Mach Zender interferometer would have identical path lengths (without the sample). However, this is not a strict requirement, since a phase shift of an integer number of wave lengths would not affect the output. If the sample has a relative phase shift of greater than one wavelength, there is no way to simply look at the detector output and determine the phase shift. However, since the samples we are examining would pass gradually into and out of the beam, by monitoring the oscilloscope output, we can determine the total phase shift by counting the number of cycles before it reversed itself (In results, we will see that the phase shift caused by our beads is almost 1 wavelength, meaning that the voltage should has a double-dip property that is approximately symmetrical).
When there is no sample in the paths, then:
To get to the detector in the path with the lenses: There is
 |
 |
 |
 |
 |
|
We never got as far as testing our device with real bacteria, given the limitations of the equipment that we had (among other problems, we did not have access to a source of cultured bacteria, nor any precise way to create suspensions of reasonable concentration).
We did, however, test our device with a solution of approximately 3.7μ polystyrene beads, which, among other benefits, provides us with a fully known system (both diameter and index of refraction) so we can compare our results to what we already know, and determine if our device is functioning.
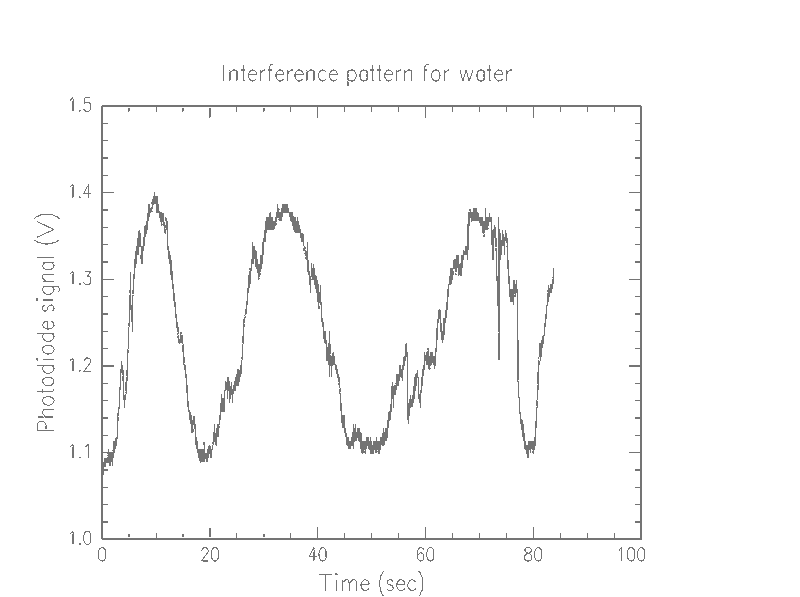
First, we were able to determine that our device is picking up the beads, and probably one at a time (we couldn't easily make lower concentrations, so we weren't able to verify this). Looking at the graphs of pure water versus the bead solution, we notice that there are a lot more spikes in the bead solution
 |
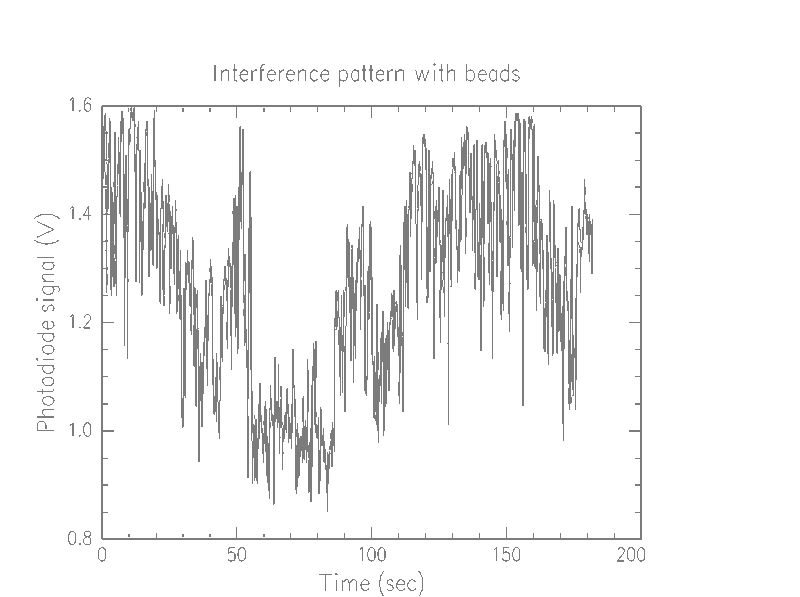 |
We beleive each of these spikes means that a bead is passing through the beam. Additionally, we notice that some of the spikes spike up, so what we are seeing is not simply attenuation due to opaque beads, but instead an interference effect.
The low frequency osciallation that jumps out at the viewer of these graphs is probably due to instability of the parts. That is, as parts are gradually jostled about during use, a phase shift is introduced. However, this effect is quite small. The time scale of one oscillation appears to be, at worst, about 20 seconds, which is much less time than it takes for a bead to pass into and out of the beam. In a much more restricted time scale, as below, the effect is negligible.
It is unclear why the dynamic range of the different media should be different. While the voltage of the water generally stays in the 1.1–1.4 V range, the beads range from .85–1.6V. One possibility is that our slides, which were simple preparations consisting of a drop of the fluid, with another coverslip simply placed on top, were of significantly different thickness. The glass itself could be a factor, with inherent manufacturing differences between any given slide.
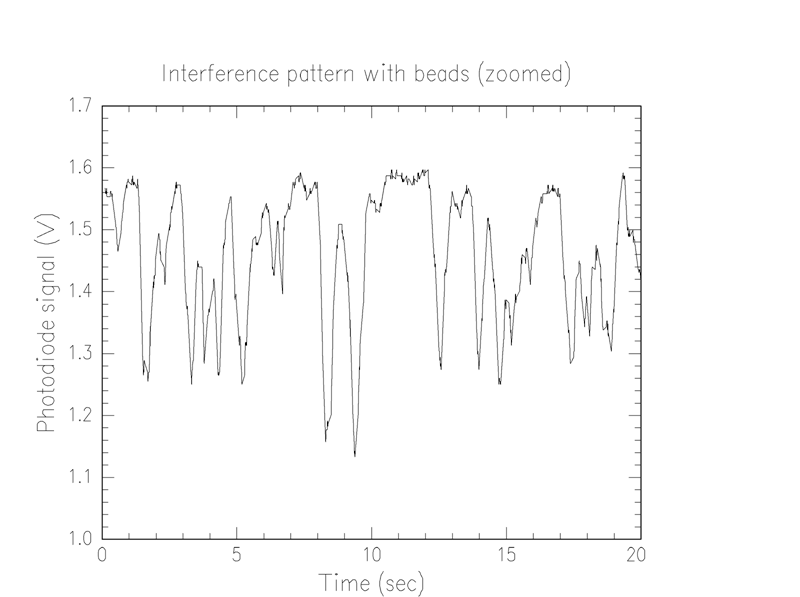
We notice that the thickest bead seems to introduce a shift of about .9 λ. Thus, assuming 3.7μ beads and a 700 nm laser, and knowing that the index of refraction of water is 1.33, we find that the index of refraction of the beads is 1.56, which is in the range of values for polystyrene.