The precise distribution of materials within a cell is essential for life, but the way the movements of cellular cargo are choreographed is largely unknown. Taking an unprecedented look at special biological tethers that help transport materials within cells, a Princeton-led team of biologists has provided the first detailed glimpse at how these tethers coordinate cargo delivery, suggesting they play a far more comprehensive role in the process than previously imagined.
While previous studies have shown that so-called "multisubunit tethering complexes" -- named for the multiple protein components they contain -- are necessary for intracellular transport, the mechanism for their involvement has been largely unknown. In results published this month in the journal Cell, the Princeton team reports a model for the Dsl1 tethering complex, which is the simplest of eight such tethers that have been identified. Their results reveal how the tether may coordinate the delivery of small "packages" of material to their cellular destinations.
"Tethering complexes have been known to represent the first physical connection between the package and its destination," said Fred Hughson, a Princeton molecular biology professor and leader of the research team. "Based on our findings, our hypothesis is that their involvement may go much deeper; these tethers may be chaperoning the entire delivery process."
Complex cells, or eukaryotes, contain a variety of membrane-bound organelles that perform specific functions, just as organs in the human body have specified jobs. Materials, such as proteins, travel throughout eukaryotic cells in bubble-like vesicles, which also are enclosed by membranes. Upon arrival at the target destination, the cargo contained within the vesicle is delivered when the vesicle membrane fuses with the membrane surrounding the target organelle, similar to the way in which two bubbles merge to become one.
As in any delivery system, it is crucial that materials are delivered to the appropriate organelle within the cell. Vesicle membranes are surrounded by special protein coats that are thought to provide some specificity about target location, akin to how an address on an envelope guides mail delivery. Additionally, there exist special proteins called SNAREs that project from the membranes surrounding vesicles and organelles. When vesicle SNAREs are in close proximity to complementary SNAREs on the surface of an organelle, they "zip up" and pull the two membranes together to enable fusion. While SNAREs are extraordinarily important, by themselves they work slowly and inefficiently, and scientists have long suspected that additional factors -- like tethers -- were needed to ensure that cargo delivery in the cell was handled smoothly and efficiently.
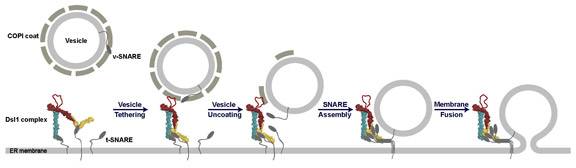
Fred Hughson and his colleagues describe a possible mechanism by which the Dsl1 tether directs the delivery of cellular cargo. With one end firmly anchored to SNARE proteins on the membrane of the destination organelle, the tether "lassos" the protein coat around a vesicle. The tether then assists in the removal of the coat and helps the SNAREs on the vesicle and organelle membranes to bind together, which brings the two membranes close enough together for fusion and cargo delivery. (Image: Hughson Research Group)
Hughson and his colleagues began by coaxing the Dsl1 tethering complex into forming crystals, which they then bombarded with X-rays. Despite the fact that the tether is an enormous molecule, containing tens of thousands of atoms, this technique allowed the scientists to determine the precise relative location of every single atom.
Their results revealed a structure some 20 nanometers tall, thousands of times shorter than the width of a human hair but 10 times the size of a SNARE, with flexible joints and a mobile lasso-like loop at one end. The lasso contains protein sequences that bind to the protein coats surrounding particular vesicles, while the other end of the tether features regions that attach to the SNARE proteins that decorate the target organelle's surface.
These structural clues enabled the scientists to posit a mechanism whereby the Dsl1 complex, anchored to SNARES projecting from an organelle's membrane, might wave back and forth in the cell and "lasso" vesicles bearing cargo for delivery to that organelle, similar to the way a sea anemone traps food in its tentacles. The tether joints might then flex, bringing the vesicle SNAREs close to the SNAREs at the other end of the tether and enabling fusion. Because the tether latches on to the protein coat, this experiment also supports previous proposals that the Dsl1 complex may accelerate the removal of the protein coat around the vesicle, which must take place before fusion and delivery can occur.
In future work, the research team hopes to explore this process further by capturing a series of "snapshots" to watch how the lasso interacts with the protein coat. Additionally, the group intends to conduct a series of test tube experiments to assess how the various features of the Dsl1 complex's structure influence the rate and efficiency of cargo delivery.
"The cell, and the traffic inside flowing in every direction, is so complex that it can be difficult to test ideas about how it works," Hughson said. "Maybe now we know enough about how tethers guide cargo delivery to reconstitute this process in a test tube, away from most of this complexity, and figure out how it really works."
Hughson's Princeton colleagues on the team include former graduate students Yi Ren, now at Rockefeller University, and Arati Tripathi, now at Harvard Medical School; David Huie, who received his bachelor's degree from Princeton in 2009; and Philip Jeffrey, manager of the Department of Molecular Biology's X-ray facility. Additionally, the research group includes Calvin Kip and Thomas Walz, both of Harvard Medical School. The work was supported by the National Institutes of Health.