|
Introduction to Frontier Research on Combustion by Peng Zhao (MAE) |
|
| |
|
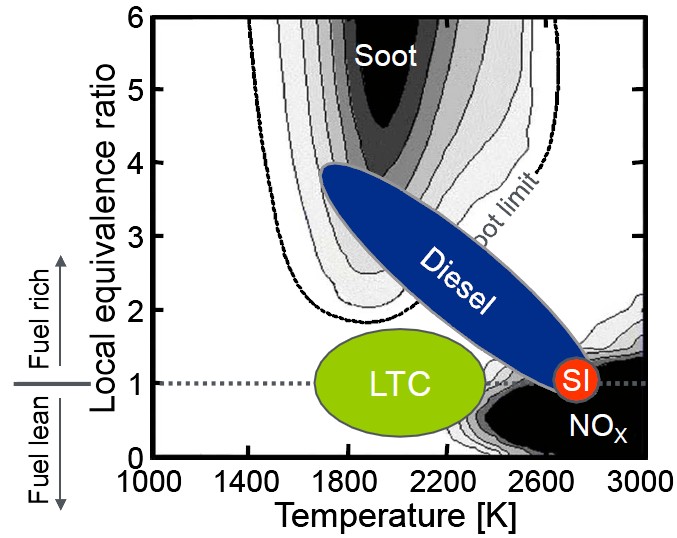
Figure 1. Working regime of low temperature combustion (LTC) technique, showing its significantly low emission for NOx and soot [2] |
|
IntroductionCombustion, initializing the civilization of human society, has developed for thousands of years. It refers to the exothermic process with light emission when fuel molecules strongly react with oxidizer, frequently oxygen. As a major way of utilizing chemical energy, it remains the most important way of providing energy in the current world, which is constrained and driven by the need to produce energy to manufacture, transport, provide heating and cooling, and generate electricity, etc. Chemical energy in the form of petroleum, coal, natural gas, and biomass taking up the largest part of the world energy, is contained in the chemical bond constructing the fuel molecules. Through combustion, chemical energy could be converted to thermal energy, and be further converted to mechanical energy through thermodynamic cycles. Historically, combustion is an application-driven scientific discipline, meanwhile driving the development of technology. In its early period of development, fire safety issues were of primary importance, together with related knowledge on flammability limits and explosions. In the 1950s, combustion research was stimulated by aero-propulsion and then by rocket propulsion. Since then the term “aero-thermo-chemistry” [1] has become synonymous with combustion. Interests in combustion-generated pollutants such as CO/NOx/SOx and soot rose in the late 1960s. The energy crisis of the 1970s stimulated research on energy saving and combustion efficiency. In the 1980s and 1990s, more interests grew in supersonic combustion, combustion emissions and the related climate science. All these technology drivers are still present in combustion research. New drivers also emerge as can be seen in the following section. Three examples as the frontier research on combustion will be introduced below, which are representative directions of modern combustion science. Frontier research on combustion energyScientifically, combustion process is the marriage of fluid mechanics and chemical kinetics, where transport processes (convection, diffusion, thermal radiation) interact with the intermediate species involved in the detailed chemical kinetics, leading to complex phenomena such as flame ignition/propagation/extinction, hydrocarbon explosion limits, flame dynamics and instability, turbulent combustion, etc. Strong specialties are needed to really understand the profound physical and chemical essence behind these phenomena. Here instead, I would like to briefly mention three recent interests in combustion field, namely, low temperature combustion (LTC) engines, alternative fuels and nanotechnology in combustion. First of all, let’s talk about the low temperature combustion engine technique, which has been intensely investigated recently. Compared with conventional diesel and gasoline engines, it has the following advantages (Fig. 1): 1) by lowering combustion temperatures through various forms of lean, or partially premixed combustion, heat loss from the cylinder is reduced leading to enhanced engine efficiency; 2) the lower combustion temperature is also useful for reducing engine-out NOx emissions, which is highly sensitive to the flame temperature according to the Zel’dovich mechanism; 3) the lean combustion process is unlikely to generate soot particles, which is a major concern for the air and environment quality. There are several versions of LTC technique, such as Homogeneous Charge Compression Ignition (HCCI), Premixed Charge Compression Ignition (PCCI), etc. Take the popular HCCI mode for example. Different from the conventional spark-ignited gasoline engines, there is no direct initiator (e.g. spark plug) of combustion. The compression ratio (> 15) of it is approaching that of the diesel engine, making ignition occurs at several local points and combustion starts almost simultaneously. This makes the process inherently challenging to control. However, with advances in microprocessors and deeper understanding of the ignition process, HCCI can be controlled to achieve gasoline engine-like emissions along with diesel engine-like efficiency. In fact, HCCI engines have been shown to achieve extremely low levels of NOx emissions without an aftertreatment catalytic converter. Moreover, the premixed lean mixture does not produce soot. It should be noted that the HCCI mode is not fuel sensitive, different fuels including gasoline, diesel, even blending liquid fuels could work in it. However, the performance of HCCI is limited by its load limit which is further strongly affected by the engine knock. Therefore, currently HCCI is used as a substitution under lower load conditions, but as a potential future engine, HCCI is a wonderful option from both combustion and environment considerations. Second, let’s talk about the combustion of alternative fuels, such as butanols, bio-diesel, etc. Before running out of fossil fuel energy on earth, we need to find alternative fuels to meet the huge need of liquid fuel (about 80 Mbpd), by certain considerations [3]. On one hand, the new alternative fuel should help to reduce the environmental impact, such as greenhouse gas, NOx, soot emissions; On the other hand, from the perspective of combustion and engine technique, the new alternative fuel should have a reasonably high energy density and suitable volatility, and more importantly, correct ignition delay and flame speed for engine cycles. For the above reasons, biofuel is a good option as future alternative fuels, which can be converted from biomass both biologically and thermochemically. There exists enough waste biomass (waste from agriculture, forestry, energy crops, algae on waste land, etc) to supply about 25% of the total demand for liquid fuels if used properly. 
Figure 2. Flame streak images of mixtures of diesel and biodiesel in a free falling droplet experiment: a) diesel b) D75B25, c) D50B50, d) D25B75, e) biodiesel The biological way is actually to use microbes to convert biomass to liquid fuels with sugar input. This process works well in aqueous media at reasonable temperatures and pressures, and generates primary products as ethanol and butanols, although the throughput is not very high. Combustion Energy Frontier Research Center (CEFRC) in Princeton University has built up an experiment database for the target fuel, including ignition delay time, species history, laminar flame speed, droplet process, and data collected from shock tubes, rapid compression machines, and flow reactors. And based on it, reaction models have been obtained to describe the chemical kinetics and predict the engine process using butanols. The thermochemical way is based on the traditional chemical processing method to convert biomass to biodiesel with extreme temperature, pressure and catalyst. Biodiesel is long chain alkyl esters, usually, methyl esters. Due to the abundant oxygen atoms in the fuel structure, the energy density is relatively lower than that of diesel; however, recent research has found that bio-diesel can substantially lower the propensity for soot emission, shown in a free falling droplet combustion experiment done by Law et al [4] (Figure 2). We could see in Figure 2 (e), the biodiesel droplet flame shows a blue chemiluminescence without the yellow emission that indicates soot formation. And from (a) to (e), the period of yellow emissions decreases with the decreasing amount of diesel in the droplet. Clearly, butanols and biodiesel are proper future alternative fuels, which can also be used as blending fuel in the current engine. Last, it would be interesting to show some interdisciplinary study in combustion research frontier, e.g. nanotechnology. Specifically, the topics of combustion synthesis of nanomaterial and the nanocatalyst in combustion propulsion systems will be briefly introduced. Recently, nanotechnology develops incredibly fast and broad interests have been imposed on nanomaterial synthesis for catalytic, electrochromic, photochromic, thermochromic considerations. Traditional methods, such as thermal evaporation method, hydrothermal synthesis, etc, have strong limitations on either extreme working conditions or the slow growth rate. However, flame synthesis method has no need for catalyst and shows a rapid growth rate, high coverage density, controllable morphology with a rather low cost. Figure 3 demonstrates a Hencken burner facility for MoO3 nanomaterial synthesis by Zheng et al [5]. Both fuel and oxidizer comes from the bottom of the burner but not premixed, so a diffusion flat flame is formed above the burner. The hot combustion intermediates and products then follow the flow, cool down at the cooling meshes with temperature control, and further react with source meshes of target material Mo, generating metal oxides vapor and going through cool meshes again, finally deposited on a changeable substrate to form nanomaterial with different morphology as shown in Figure 3. The key of this experiment is rather simple: control the equivalence ratio of the combustion process and the source and cooling temperature to control the metal vapor concentration and deposition rate. The background for nanoparticle catalyst lies in the requirement of scramjet engines, shown in Figure 4. Due to the high Mach number of the incoming flow, the flow in the scramjet burners after a series of oblique shocks is supersonic, giving extremely short residence time (in the order of milliseconds) for fuel injection, vaporization, mixing, ignition and burnout. So scientists are trying to investigate the potential to facilitate ignition by using nanoparticle catalysts: for one thing, its large surface to volume ratio can provide more sites for surface reactions; for another, there is no need for support materials using nanoparticle catalyst, further eliminating the related influence. 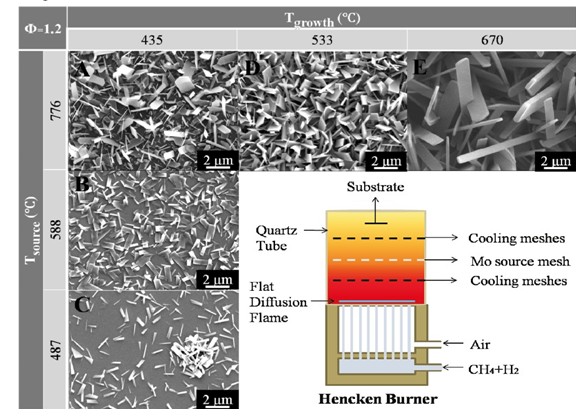
Figure 3. Hencken burner for nano MoO3 synthesis and different morphology 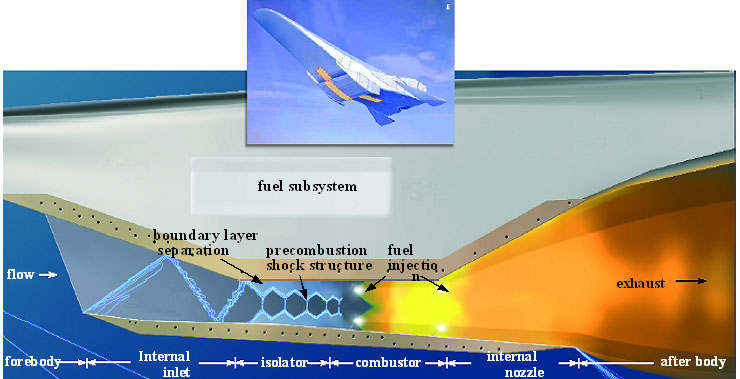
Figure 4. Structure of a scramjet engine [6] Wang et al [7] then develops a flow reactor technique with in situ generated palladium nanoparticles (Pd). For the same experimental conditions non-catalytic, homogeneous ignition is observed at a furnace temperature of 1123 K, whereas ignition of the same mixture with the nanoparticle precursor compound (Pd(THD)2) happens at about 973 K, showing the advantage of nanoparticle catalyst. By using scanning mobility particle sizer, transmission electron microscopy and X-ray photoelectron spectroscopy analysis of particles collected at the reactor exit, they have confirmed the formation of Pd/PdO nanoparticles and the log-normal size distribution. Then they construct a mechanism for methane catalytic ignition by Pd/PdO catalyst, based on which, their calculation finds that nanocatalyst shortens the ignition delay time of methane/air mixtures by about two orders over a wide range of conditions. SummaryTo summarize the major points of this article, the development of combustion energy is going toward the directions of new engine technique, new alternative fuels, and new interdisciplinary field study. Considering the interdisciplinary nature of combustion itself, there is a huge possibility of the coupling of other fields, e.g., material science, biology, etc, and combustion in the future. With combustion still being the major energy source, other energy utilization methods should be strongly encouraged and explored so that more future energy options could be developed. Reference[1] Jozef Jarosinski and Bernard Veyssiere, combustion phenomena, Taylor and Francis Group, 2009
|
|
Copyright 2012 by Princeton University China Energy Group. All rights reserved. |
