Pyrochlore
Ca2Nb2O6F
Ca2Nb2O6F (download cif) is the nominal pyrochlore mineral. The general formula for Pyrochlore is A2B2O7, or more accurately: A2B2O6O'. In the case of nominal pyrochlore, F occupies the F' site. Pyrochlore is highly symmetric, and is face-centered. The easiest way to begin to visualize the structure is by looking at the unit cell with only the F and Ca atoms (the A and O' atoms of the general formula):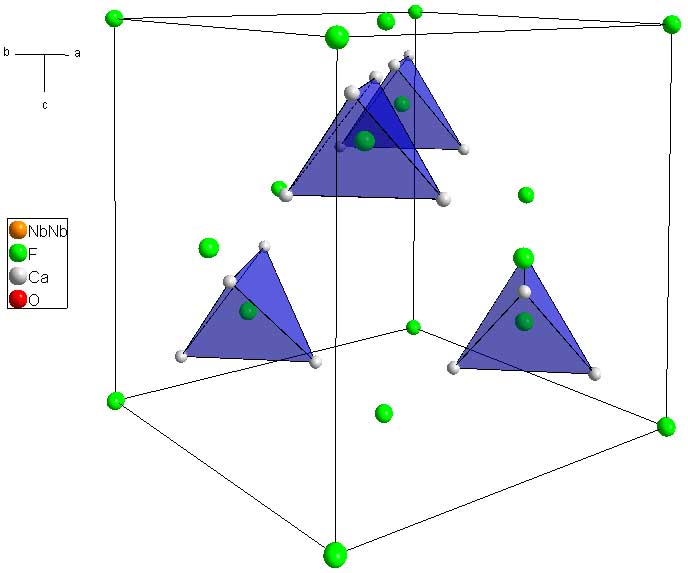
First you'll note that the F atoms sit on all of the FCC lattice points. In addition to these, there are for more flourine atoms which sit in tetrahedra holes created by the Ca atoms. You'll also note that the Ca atoms sit on a kagome net meaning that they sit on the corners of tetrahedra which share corners in all 3 dimmensions.
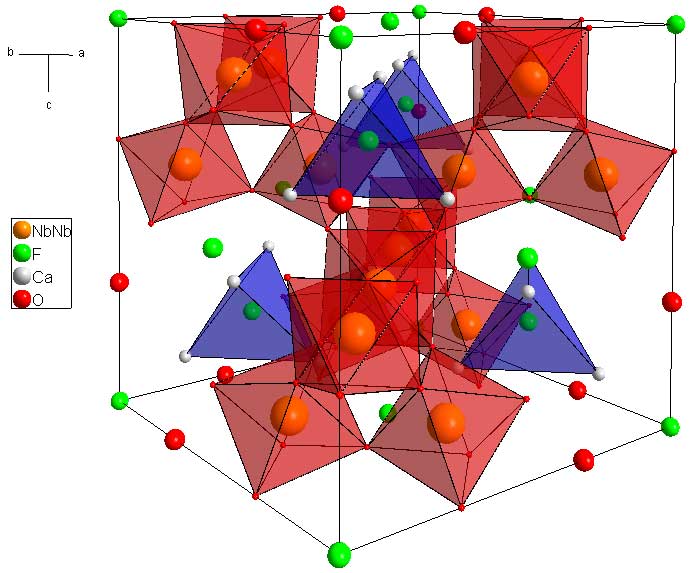
The next step is to add the Nb atoms

You should notice that the Nb atoms, which sit on the B site, also form tetrahedra. They sit on their own Kagome net, and the A a B cations form two interwoven Kagome nets. However, there is no atom occupying the tetrahedral hole formed by the B atoms. The other difference between the A and the B site is their level of oxygen coordination. The A cations have an 8 fold coordination whereas the the A have octahedral coordination. In the last picture I show the NbO6 octahedra. All of the Oxygens sit on the corners of these octahedra.
back to structure index