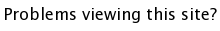Rutile Structure and Derivatives
Rutile
TiO2 (titanium dioxide, download cif) is the nominal rutile structure. It can be thought of as infinite columns of edge sharing TiO6 octahedra. In addition, each edge-shared oxygen is corner-shared with an adjacent infinite chain. As such, each Ti is coordinated to 6 oxygens (by definition for octahedral coordination) and each oxygen is coordinated to 3 titaniums, 2 within a column, and 1 within the adjacent column.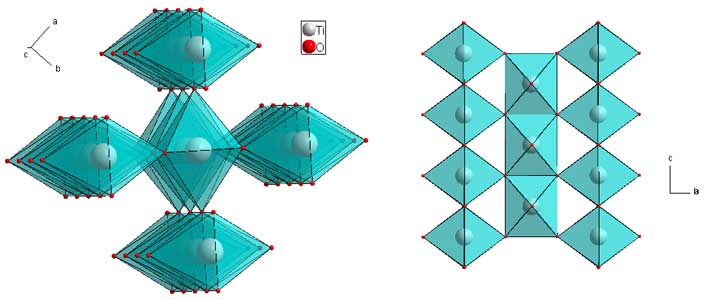
Rutile Derivative: ABO4, ordered and disordered
Two different metals can replace Ti in the rutile structure giving a new formula of ABO4, keeping the metal-oxygen ratio. If the metals are disorded on the site, then the crystallographic description remains the same, with 50% of each metal on the Ti site. While this is most often the case, the metals can order. The image is the reported structure for CoReO4 (download cif)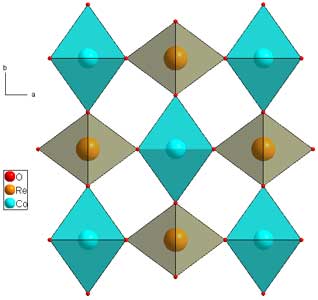
AB2O6, tri-rutile structure
In a 1-2-6 ration, the metal cations will have the same basic skeleton as rutile, but the cations will order is such a way as to triple the c-axis, i.e. the axis pointing along the infinite edge-sharing columns. FeTa2O6 (download cif) is shown below as an example.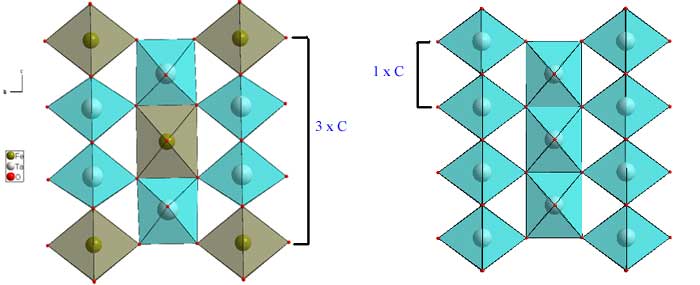
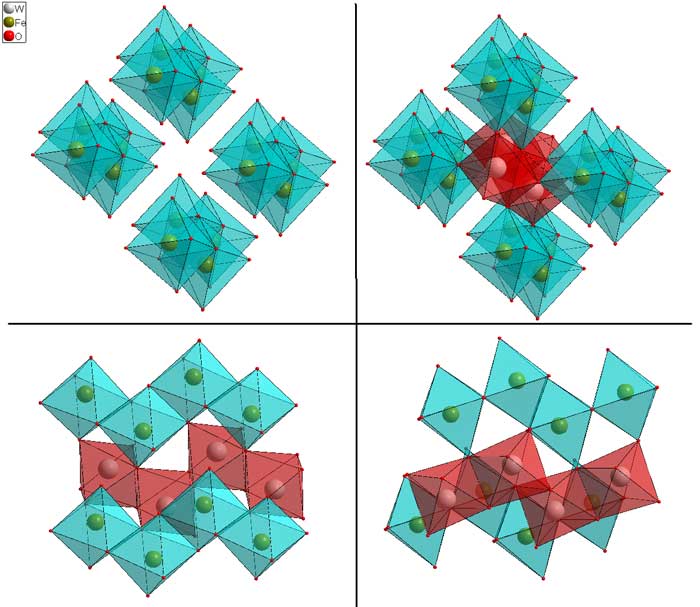
skew-edge sharing, alpha PbO2 / columbite structures
In some cases, the MO6 octahedra will share skew edges. In such a case the infinite edge-sharing columns become zig-zagged instead of straight. However, the edge-shared oxygens still share 1 corner with an adjacent chain and in that sense the structure is the same as rutile. In the figure below, FeWO4 (download cif) is used as the example. This structure is a little difficult to visualize, so using a structure viewer is useful (use the cif file). In the upper left, only the FeO6 octahedra are shown. In the upper right, the WO6 octahedra are added, but the structure orientation is the same. Two simplified versions from the side are shown in the lower panels.In the case of ABO4, the A and B cations are ordered on the two different sites typified by Fe and W. In the case of AO2, (e.g. alpha-PbO2, download cif), the Fe and W sites are equivalent, and occupied by the same cation.
back to structure index