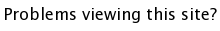Spinel part 2
Spinel, part 1
MgAl2O4, Spinel
Spinel, MgAl2O4 (download cif), is a rare gemstone that was used to imitate rubies. The structure has face-centered cubic symmetry, and is a little heavy to understand. The general formula is AB2O4. The oxygens form an fcc lattice. The A cations occupy tetrahedral holes, and the B cation occupy octahedral holes. It then follows that 1/8 of the tetrahedral holes are occupied, and 1/2 of the octahedral holes.The first way to think of the spinel structure is the array of Al atoms. They sit on the corners of tetrahedra which share corners infinitely in 3 dimmensions, an array known as a Kagome net. The image below shows only the Al atoms sitting on the corners of tetrahedra.
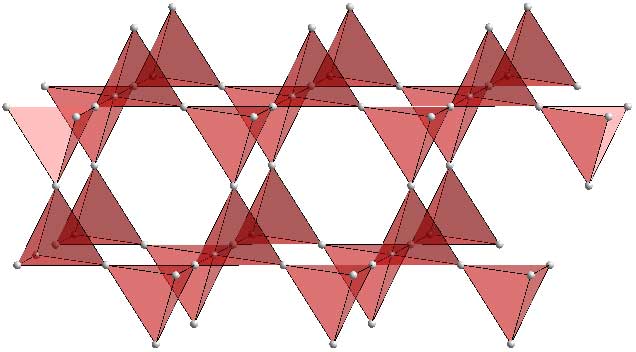
Now we add the Mg atoms.
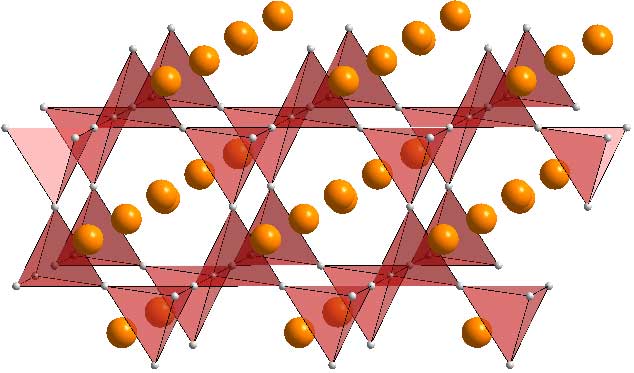
As we said above, the Mg atoms sit in tetrahedral holes, so lets show the MgO4 tetrahedra.
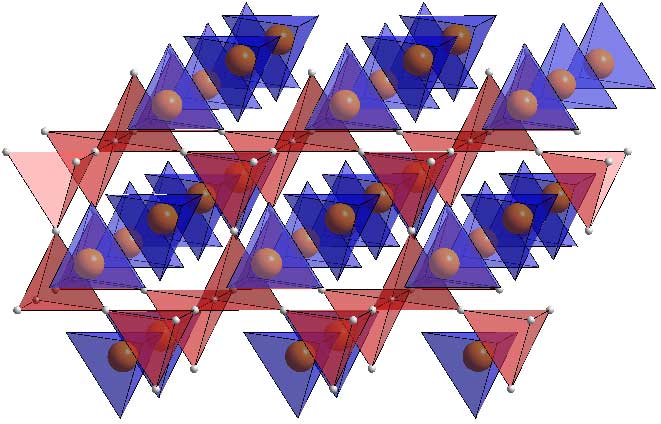
How's that for clarity?
Let's consider this from another point of view
Spinel part 2
back to structure index