![]()
Feature - January 28, 1998
Scientists look to seashells and cell membranes
to create new materials
by Billy Goodman '80
 The humble abalone, a marine mollusk and delicacy, builds a strong
shell from unspectacular materials: calcium carbonate and protein. By
tying thin layers of calcium carbonate -- which in another form makes
chalk -- together with a protein, the abalone builds a laminate that is
strong and durable.
The humble abalone, a marine mollusk and delicacy, builds a strong
shell from unspectacular materials: calcium carbonate and protein. By
tying thin layers of calcium carbonate -- which in another form makes
chalk -- together with a protein, the abalone builds a laminate that is
strong and durable.
The structure of abalone shell was elucidated by biologists, but a decade ago it attracted the attention of Ilhan A. Aksay, a professor of chemical engineering and an expert in the processing of ceramics, who was then at the University of Washington. Over the years, he has been funded by the U.S. Army to develop lightweight armor and has made and patented some ceramic-metal composites. (A boron-carbide/aluminum composite that Aksay helped develop was used on U.S. military vehicles in Bosnia.) When he learned about abalone shell he thought, "Why not make such composites laminated?" He and his coworkers did, and patented a laminated version of the boron-carbide/aluminum material that is 25 percent stronger than the unlaminated version.
Aksay is one of a number of Princeton scientists and engineers who are studying the tricks of abalone and other animals and plants to fabricate new materials with properties that improve on other man-made materials. Princeton's materials scientists do not, it should be emphasized, want to recreate abalone shell in the lab. If we just did that, says Professor of Chemistry John T. "Jay" Groves, "we would only have a clam shell." Instead, more than a half-dozen Princeton researchers from almost as many departments are trying to understand the processes that living things use to create the materials of life, and then to adapt these processes for improving man-made materials. "If we learn the rules," says Groves, "we can make anything."

Ilhan Aksay, left, and Jay Groves: materials scientists taking inspiration from biology.
Groves and Aksay are learning the rules as members of the Princeton Materials Institute. Started in 1990, PMI is the university's attempt to unify the materials sciences at a time when some are predicting that an Age of Materials is at hand.
Princeton does not have a materials-science department and in some sense can be said not to have any materials scientists, either. At least, not in the conventional sense of scientists who have earned degrees in materials science. Princeton never seriously considered starting a department of materials science, preferring instead to promote interaction of scientists in existing departments. PMI is a hybrid between a department and a center. It occupies its own modern building -- Bowen Hall -- on Prospect Avenue, on the same block as the Engineering Quadrangle. In that way, it is a bit like a department. PMI can also make joint appointments of faculty, also department-like. But in serving as a gathering place for faculty from many departments interested in materials science, and in providing a home for expensive equipment to be shared, PMI operates like a center. Moreover, students cannot earn degrees from PMI. Undergraduates may earn a certificate. Graduate students do materials-related research with an affiliated faculty member, but get their degree from their professor's department.
ONE TOUGH MOLLUSK
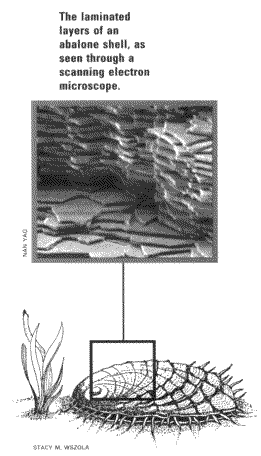 The key to the abalone shell's toughness, according to Aksay, is in the
details of its structure, which has been worked out over the last few
years by researchers at Princeton, the University of California at Santa
Barbara, and elsewhere. Magnified 300,000 times with an electron
microscope, the shell looks like a brick wall, with calcium carbonate
"bricks" alternating with a protein "mortar." Despite the essentially
brittle nature of the calcium carbonate, the shell is extremely strong and
less brittle than man-made ceramics, due to its laminated structure. That
lamination helps prevent cracks from propagating, in somewhat the same way
that a braided rope does not fail when one strand breaks.
The key to the abalone shell's toughness, according to Aksay, is in the
details of its structure, which has been worked out over the last few
years by researchers at Princeton, the University of California at Santa
Barbara, and elsewhere. Magnified 300,000 times with an electron
microscope, the shell looks like a brick wall, with calcium carbonate
"bricks" alternating with a protein "mortar." Despite the essentially
brittle nature of the calcium carbonate, the shell is extremely strong and
less brittle than man-made ceramics, due to its laminated structure. That
lamination helps prevent cracks from propagating, in somewhat the same way
that a braided rope does not fail when one strand breaks.
Aksay is an enthusiastic proponent of "biomimetics" -- looking to biology for inspiration for new materials and materials-processing techniques. "When people ask me if I needed to study biological systems to invent laminated ceramic-metal composites," he says, "I tell them, no, I might have come up with the idea anyway, but studying biological systems helped get me there faster."
Biomimetics is a fertile area in materials science today, and a strength of PMI. Aksay became interested in the field more than a decade ago, when he read a book by Duke University zoologist Stephen Wainwright and others titled Mechanical Design in Organisms. About the same time, he and colleagues were working on ways to make materials that looked -- microscopically -- like abalone shell. They never intended to simply duplicate the shell material, since they could start off with much better raw materials than calcium carbonate. "Just because we mimic nature, doesn't mean we make materials exactly the same way," he says.
In an interview in his Bowen Hall office, Aksay reveals a straightforward motivation for much of his work: "The excitement of making a new material is a driving force for my research." And a material, to Aksay, is something you can make gadgets out of, something you can get your hands around. Materials can range from crystals, with very precise and ordered structures, to amorphous solids such as glass, to liquids with some ordered structure called liquid crystals.
If Aksay's office and nearby laboratory were a material, they would be a crystalline solid, so neat and ordered are their contents. In a typical scientist's office, journals and papers are strewn about, often obliterating any glimpse of the desk. Aksay's are in square stacks. Books and notebooks on his shelves line up with micrometer precision. Meanwhile, Aksay is trying to make materials in which he controls the structure down to the nanoscale. (Nanoscale refers to dimensions from roughly one nanometer to one micrometer. A nanometer is one-billionth of a meter; a micrometer, or micron, is one-millionth of a meter. At the scale modern materials scientists work, 100-micrometer-thick human hairs are like the cables of a suspension bridge.)
There are two paths to capturing the nanoscale, says Aksay. One can essentially carve out of a hard material a structure on a tiny scale. For example, an experimental technique known as e-beam lithography uses beams of energetic electrons to make a pattern in a hard substrate, rendering possible computer chips that are 100 times smaller than current commercial versions. But what about working with soft materials, as nature does? Materials scientists are in fact looking to living things for more than just materials to model -- they would also like to adapt the assembly process that nature uses, known as "self assembly." Simply put, the building blocks of living things -- organic molecules -- come together on their own, with some direction from genes, and form bonds that lead eventually to biological structures. The entire process of development, from a fertilized egg or seed to an adult organism, is self-assembly.
RUNNING A FILM IN REVERSE
"Amazing," is chemist Groves's reaction to such examples of self-assembly and development as a seed growing into a tree. "It works," he says, "because a seed contains all the information in itself for how to make molecules, which then arrange themselves. That's what we mean by self-assembly."
In the course of several interviews in his comfortable office in the chemistry department -- which, though neat, is distinctly less orderly than Aksay's: call it a liquid crystal -- Groves returned again and again to a building analogy. When people erect a building, he says, they first make the bricks, then a laborer arranges the bricks, slathering on mortar as he goes. But nature has no builder, says Groves. Instead, nature's way of making a building looks more like a film of an explosive demolition run in reverse, with rubble rising up miraculously to form a building. "Nature doesn't build brick by brick, it just erects," he says.
One biological material that has drawn Groves's attention, as well as that of several other Princeton materials scientists, is the biological membrane. Found around all cells and also around the cell's nucleus and many organelles within the cell, biological membranes are not just a single material, but a class of similarly constructed materials. The typical membrane, introduced early in a general biology course when the topic turns to cells, is a double layer of lipids with protein molecules floating among the lipid chains. Each lipid molecule in the membrane has two ends, one that is hydrophobic (water-hating) and one that is hydrophilic (water-loving). When exposed to water, or watery fluid between and within cells, the lipids spontaneously form structures that are stable energetically, with their hydrophilic heads facing the water and hydrophobic tails facing each other in the interior of the membrane. (This "hydrophobic effect" was recognized and named by Professor Emeritus Walter Kauzmann of the chemistry department.) In other words, lipid bilayers self-assemble.
CREATION WITH A SMALL "C"
Groves and his team of 15 postdoc toral fellows, graduate students, and un dergraduates work in a large, well-equipped laboratory to understand how biological membranes are made and to build materials similar to them. Groves emphasizes that he approaches materials science from the science rather than the engineering end of things. Unlike Aksay, he is not motivated by visions of an end product. Nevertheless, the process of science for Groves includes both discovery and creation. Discovery, he says, involves trying to determine "not only what nature has done in detail, but also what are the general strategies that nature has evolved." Once he has discovered what nature has wrought, Groves muses about building "one of those things myself. That's creation with a very small 'c'."
He and his students are at work on a half-dozen or more projects, all sharing a common intellectual framework: understanding and orchestrating complex molecular interactions and associations. "Can we create large-scale organized structures using the processes that cells use?" he asks. The answer to this question is clearly yes. And while Groves is motivated by basic curiosity, much of his work has potential applications.
For example, he has studied structures that might be substitutes for conventional imaging agents used in the medical diagnostic test called MRI, or magnetic resonance imaging. Now, patients getting an MRI are loaded up with several grams of a heavy metal to allow the device to create a picture of soft tissue. Because heavy metals are poisonous and may not all wash out of the body, medical technologists wonder how to make imaging agents without heavy metals. Iron might be a good substitute, since the body already uses it and has evolved mechanisms to deal with it. Furthermore, tiny magnets, just a few nanometers in diameter, might be sufficient. If scientists could encase such tiny magnets in a membrane, they might make good imaging agents. Some bacteria have already done the job, growing magnets -- in the form of magnetite, a magnetized iron used in compasses -- in tiny sacs, or vesicles, inside their single-celled bodies. (They use the magnetite to tell up from down in the soil. Transplant northern-hemisphere bacteria to the southern hemisphere and they go in the opposite direction.)
Groves's lab has produced such sacs and grown magnetite crystals inside them. Because they are made of phospholipid membranes, the sacs ought not to cause an immune response in a recipient. Furthermore, if some sacs broke and released their iron, it would simply be a tiny addition to a person's natural store of iron. The sacs are so tiny, Groves says, that when added to a glass of water they don't cloud it up at all.
Groves acknowledges that "we're a ways from putting them in a person." Animal studies are needed first. Such experiments, followed by clinical trials perhaps, are for others to worry about; Groves is satisfied with the opportunity to test his understanding of membrane structure and function. He is concerned that applications not be oversold. "You have to sell science on its intrinsic value," he says, "not sell individual solutions, in part because such solutions may be years off and in part because it creates a narrow-mindedness of thought."
Princeton's official entry into the materials sciences coincides with a flourishing of interest in the field and the development of new analytical techniques. The field is hot and the reason, says Groves, is that "until now, we have focused on what is -- such as the structure of wood, or of a tooth -- and now we are asking about putting together things that nature has not thought of." There is no intrinsic boundary to this process. Presented with a goal, materials scientists use their knowledge of synthetic and biological materials to try to meet it.
Billy Goodman '80, a science writer who lives in Montclair, New Jersey, wrote about the Department of Molecular Biology in the February 9, 1994 PAW.