Microgravity Droplet Combustion
Detailed Kinetic Modeling of N-Alkane Droplet Combustion
 Research Summary
Research Summary
There is a critical need for partially-reduced, high temperature, n-alkane kinetic mechanisms which accurately reproduce a wide variety of kinetic and flame observations, which are sufficiently small for use in transient, uni-dimensional reactive flow computations, and which can serve as skeletal models for developing even smaller reduced representations for multi-dimensional, time-dependent computations and rate-ratio asymptotic studies. Such a mechanism has been developed for n-heptane (Held, Marchese and Dryer, 1997), and the technique utilized can easily be ex-tended to consider larger alkanes and mixtures.The mechanism considers fuel thermal decomposition reactions and realistically accounts for the site-specific abstraction of hydrogen atoms and the subsequent heptyl radical b-scission processes. Validation against kinetic data from stirred reactors, a flow reactor, shock tubes, and laminar flames were first performed. The mechanism was then incorporated in transient, spherically symmetric, isolated droplet calculations using a time-dependent, finite element, chemically reacting flow model (Marchese, 1996) which also considers detailed molecular transport. No kinetic or gas-phase transport parameters were modified to achieve agreement with experimental results. The only free parameter in the model is that utilized to approximate enhanced mass transfer due to internal liquid-phase motion (Marchese and Dryer, 1996). The development was motivated primarily by DCE experiments, first flown aboard Space Shuttle Columbia (STS-83) in April, 1997.
 Modeling Results - Pure n-heptane
Modeling Results - Pure n-heptane
The entire combustion history (ignition, pre-mixed/diffusive flame transition, droplet heating, vapor accumulation, quasi-steady combustion, and extinction /burn out) were calculated. Ignition delay experiments of Faeth and Olsen (1968) were simulated and, during quasi-steady combustion, the calculated droplet flame structures were compared with calculations using an earlier mechanism proposed by Warnatz. For the combustion of small (less than 1 mm) heptane droplets in air at 1 atm, the model predicts very small extinction diameters which is consistent with the results of Hara and Kumagai and Yang and Avedisian who observed either burn-out (i.e. extinction diameter too small to measure) or extinction diameters of less than 100 microns.The DCE experiments consider large (1 - 5 mm), single component (n-heptane) droplets burned in He/O2 oxidizing environments of various O2 content at atmospheric and sub-atmospheric pressures. Reduced pressures and inert substitution reduce sooting propensity and increase extinction diameters, thus enabling accurate experimental measurements which are not perturbed by sooting effects. The following figure compares the species and temperature calculated using the new mechanism and the semi-empirical mechanism of Warnatz and reveals a dramatic difference in the C2 and C3 intermediate distributions.
In terms of maximum mass fraction, the new mechanism predicts an increase in C2H4 and C2H2 production by factors of 8 and 3, respectively, and a 4-fold decrease in maximum C3H6 mass fraction. However, both mechanisms predict a similar flame position (location of maximum flame temperature) and similar distributions of major product species (O2, CO, CO2, H2O).
The latter result is consistent with the diffusion flame modeling study of Bui-Pham and Seshadri where temperature and major species measurements were reasonably reproduced using the Warnatz mechanism. For the intermediate C2 and C3 species, the new mechanism also appears to be in better qualitative agreement with the experimental measurements of Hamins and Seshadri. There, the measured ordering of these compounds was C2H4 >C2H2 >C3H6.
The following figure is a plot of the fuel destruction rate (vs. normalized position) due to each reversible reaction involving the fuel for the same conditions as the previous figure. Also included in the plot is the total fuel destruction rate.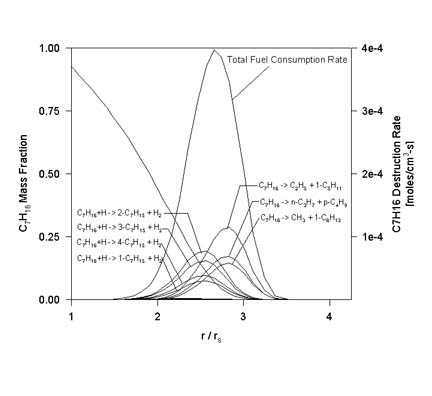
At these conditions, the reaction responsible for the largest percentage of fuel consumption (23.96%) was thermal decomposition:
The second-leading consumption reaction (17.36%) was the H-atom abstraction via H radical attack leading to 2-heptyl:C7H16 -> C2H5 + 1-C5H11
In overall terms, fuel thermal decomposition accounted for 50.2% of all the fuel consumption, while all of the fuel + H radical attack reactions accounted for an additional 46.6%. The only other class of reactions responsible for greater than 1% of the overall fuel consumption were the fuel + CH3 radical attack reactions.C7H16 + H -> 2-C7H15 + H2
These observations are important to further reduction of the mechanism and emphasize the significance of decomposition in large-hydrocarbon diffusion flames. The effect of radiation on n-heptane droplet combustion is also being examined. The figure shows a comparison of the instantaneous burning rate of a 3 mm n-heptane droplet in 1 atm air between calculations with and without radiation. As the figure suggests, the model predicts that the effect of radiation is even more pronounced in the case of heptane than for methanol.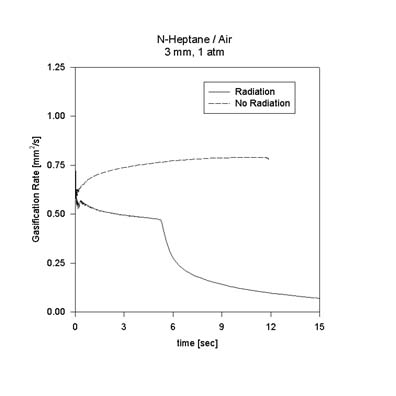
Calculations for the actual DCE test points are currently underway. For the DCE test point D103515 (1 atm, 35% O2, 65% He, 1.5 mm) the effect of radiation loss on extinction diameter appears to be negligible. However, the burning rate is consdierably reduced by the presence of radiation as initial droplet size increases. Additional results will be added to this page as they become available.

 Modeling Results - Heptane/Hexadecane Mixtures
Modeling Results - Heptane/Hexadecane Mixtures
It is possible to use the principles described Held, et al. (1997) to generate a similar sub-mechanism for larger n-alkanes such as n-hexadecane. The H-atom abstraction reactions yield 8 different hexadecyl radicals and 16 new 1-olefin species. An additional 8 species (1-alkyl radicals) result from the C-C bond cleavage thermal decomposition reactions. In total, the resulting mechanism, however, contains practically double the amount of species and is thus too large for the transient droplet combustion calculations of interest here given the current state of computational speed.
Applying the assumptions outlined in Held, et al. (1997) (i. e. infinitely fast b-Scission of all alkyl radicals greater than C2H5), the 8 C-C bond cleavage thermal decomposition reactions are shown to result in only 2 different product distributions. Thus, the entire thermal decomposition of the fuel can be formulated as 2 overall reactions:
n-C16H34 -> 2CH3 + 7C2H4
n-C16H34 -> 2C2H5 + 6C2H4.
The rate constants for the two overall reactions are determined by establishing 3 parameter fits for the sum of the rate constants for the odd and even reactions above. The rate constants for the 8 decomposition reactions are estimated from Dean (1985).
The entire series of H-atom abstraction reactions are approximated by the following 8 overall reactions as:
n-C16H34 + X -> 7C2H4 + C2H5 + XH,
and
n-C16H34 + X -> C3H5 + 5C2H4 + C2H5 + CH3 + XH
where X refers to the radicals H, OH, O and CH3. The product distributions of the above reactions are not arbitrary. The product distribution of the first reaction corresponds to the distribution due to abstraction of a one of the 6 primary H atoms and subsequent b-Scission of the resulting 1-C16H33 radical. The product distribution of the second reaction is developed by considering the final products of the 28 secondary C-H sites assuming that the large 1-olefins thermally decompose solely via cleavage of the C-C bond one removed from the double bond.
The following are plots of the diameter-squared and instantaneous gasification rate for a 1 mm n-heptane/n-hexadecane droplets in an air at 1 atm with a liquid mass Peclet number of 2. At this value of Peclet number, the system behaves in a manner closer to that of the distillation limit, than the diffusion limit. As the figure suggests, after the initial transient droplet heating, the pure n-heptane droplet closely exhibits classical d2-law droplet combustion behavior as the gasification rate is nearly constant over the majority of the droplet lifetime. For mixtures of n-heptane/n-hexadecane, the gasification rate varies continuously during the droplet lifetime. The gasification rates of the 25% and 50% hexadecane droplets reflect three distinct periods of quasi-steady combustion. The first period constitutes the preferential gasification of n-heptane, which is the more volatile component. The second period begins when the n-heptane has been depleted from the droplet surface. Once this occurs, the droplet heats up toward the boiling point of n-hexadecane resulting in a de-crease in gasification rate. During the final period the gasification rate increases as the droplet has been depleted of n-heptane.
The figures show that increased initial hexadecane content results in decreased initial burning rate and earlier onset of the secondary heating period. It is further noted that, even in the case of low Peclet number, the model does not predict a substantial plateau during the secondary heating period, as has often been observed in experiments conducted in normal gravity. This result is also consistent with experiments of Yang and Avedisian (1988) in which the secondary heating period was not observable within the temporal and spatial resolutions available.
It is noted from the above figure that the model predicts that, for small alkane droplets in air at 1 atm, the extinction diameter is too small to resolve. This result is somewhat consistent with the results of Yang and Avedisian (1988) and Hara and Kumagai (1990). In these experiments, extinction of the droplet flame at a finite droplet diameter was either observed at very small diameters (less than 100 mm) or not observed at all. For example, in the experiments of Yang and Avedisian (1988), pure n-heptane and pure n-hexadecane droplet flames appeared to extinguish at a finite diameters, while mixtures of heptane/hexadecane did not exhibit extinction. In the experiments of Hara and Kumagai (1990) pure n-heptane droplets very rarely exhibited flame extinction at finite, observable diameters. For larger initial diameter droplets, extinction may indeed be observed since, as will be discussed the effect of radiative heat loss becomes significant with increased initial droplet diameter.
Flame Extinction During the Secondary Heating Period
Finally, the effect of initial pressure on combustion and extinction of heptane/hexadecane droplets is explored. The final figure is a plot of the diameter-squared vs. time for the combustion of 25% hexadecane droplets in 1.0, 0.5, and 0.176 atm air. For a pressure of 0.176 atm, the model predicts the flame to extinguish during the secondary droplet heating period.Reduced chemical kinetic rates due to decreased pressure result in increased reactant leakage and lower flame temperature during the initial burning period. As the liquid n-heptane becomes depleted, the flame temperature drops further and, before the liquid droplet can be heated to a level high enough to liberate significant levels of gaseous hexadecane, the flame extinguishes. Although not previously identified as such, it is quite possible that flame extinction during the secondary heating period has recently been observed experimentally in a 5.2 mm initial diameter heptane/hexadecane droplet experiment conducted aboard FSDC-1 (Dietrich, et al., 1996). In this case, the experiments were conducted in 1 atm air, but due to the large droplet diameter, it is theorized here that radiative heat loss resulted in an initial flame temperature much lower than expected. Further decrease in the flame temperature during the secondary heating period was sufficient to extinguish the flame. The remaining large, hot droplet ( » 500 K) then proceeded to vaporize as it slowly cooled.
 Science Support for Space-Based Droplet Experiments
Science Support for Space-Based Droplet Experiments
The experiments and modeling conducted in the Laboratory of Prof. Dryer at Princeton provide scientific support for droplet combustion research in conducted at NASA-Lewis Research Center in the 2.2 second and ZGF 5.18 second droptowers, as well as aboard the Space Shuttle. The first type of shuttle experiment is termed the Fiber Supported Droplet Combustion(FSDC) Experiment. This experiment is designed to be operated within the Glove Box Facility. The FSDC experiment was first flown as FSDC-1 aboardUnited States Microgravity Laboratory-2 (USML-2) launched on October 20, 1995 on Space Shuttle Columbia mission STS-73. A second fiber-supported droplet combustion experiment, FSDC-2,was to operate on Microgravity Science Laboratory-1 (MSL-1), launched aboard STS-83 Space Shuttle Columbia mission launched April 4, 1997. However, the mission was curtailed from 16 days to approximately 5 days by malfunction of one of three fuel-cell systems aboard the orbitor. The operation of this experiment was precluded altogether by the shortened mission.
The second type of Shuttle experiment supported by Princeton is called the Droplet Combustion Experiment (DCE). The first flight of this facility experiment was as part of MSL-1 aboard the STS-83 launched April 4, 1997. This experiment successfully operated during the curtailed mission to achieve the first observations of free-floating, isolated droplet combustion in space. A re-flight of this mission is presently scheduled for July 1, 1997.
A re-flight of the MSL-1 mission was launched July 1, 1997. As of the update of this page, both FSDC-2 and DCE experiments are operating well. Over twenty five successful FSDC-2 experiments have been performed thus far, using methanol, 30% water/70% methanol, 15% water/85% methanol, ethanol, 4% water/ 96% ethanol as fuels. More than 20 DCE experiments have been performed with a about the expected probability of success (> 50%). See the STS-94 Summary Page for further information.
Future flights of both experimental configurations for studying droplet combustion are currently being planned, and droplet combustion research is also continuing in the NASA-Lewis drop towers.
