...because... 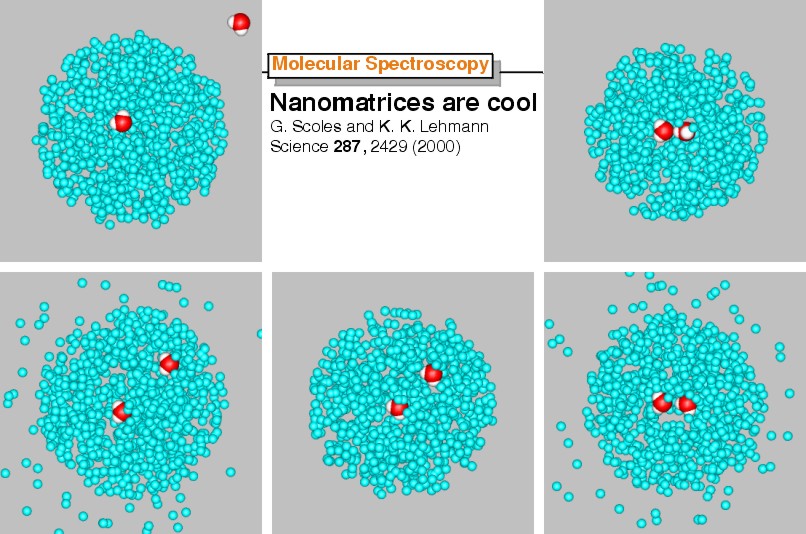 |
| WHY? | HOW? | WHAT? | WHAT NEXT? |
...because...  |
This series of images (counterclockwise, starting from top left corner) shows a rendition (not to scale) of the sequence of events culminating in the formation of a cold molecular complex (in this case the H2O dimer) inside a He droplet.
Due to the low temperature and cooling during complexation, very unusual clusters can be synthesized in this way.
Helium droplets are superfluid; they can capture atoms/molecules (even large ones) and cool them to 0.38 K, without impeding their motion (translation and rotation) within the droplet; they can be used to form complexes by multiple capture. Because the release of binding energy tends to destroy the droplets, weakly bound complexes survive, while strongly bound ones are lost from the beam. Most of these facts were unknown just a few years ago, and a good fraction of them was discovered in our laboratory. The two questions we're asking now are:
Imagine you land on a liquid planet, something like a huge sphere made
entirely of water. Gravity pulls you towards the center of the planet and---depending
on whether your density is higher or lower than that of water--- you either
get inside or sit on a small dimple on the surface of the planet. You also
have some kinetic energy, which means you do not stay put, but rather you
are ---again depending on your density, either randomly moving near the
center of the planet, or floating on its surface.
The same happens to atoms and molecules ("dopants") captured by
He droplets, except that van der Waals forces are involved instead of gravity.
In complete analogy to the planet picture, dopants still have the "option"
of going inside or remaining on the surface of the droplet. What counts
here is no longer the density, but rather the van der Waals interaction
of the dopant with He: if it is stronger than the He-He van der Waals interaction,
then the dopant will solvate and go inside, otherwise it will remain on
the surface. The residual thermal kinetic energy (remember the temperature
is low but finite: 0.38 K) will make the dopant rattle inside, or surf outside.
So far, only alkali (and some alkaline earth) atoms have been observed to
reside on the surface; everything else is solvated inside.
Now, at 0.38 K He is no ordinary liquid: it is superfluid, which in our
case means the motion of the dopant is minimally hindered. In particular:
(1) molecular rotation is not damped (unlike in any other liquid, rotationally
resolved spectra can be observed even for strongly asymmetric molecules)
(2) two or more dopants in the same droplet will quickly find each other
and form a van der Waals bound complex, whose geometry might be determined
by the presence of barriers in the dopant-dopant interaction potential. One of the main goals in the study of the mechanisms
of chemical reactions is the stabilization of unstable species. Helium nanodroplets
have turned out to be an ideal medium to reach this goal. At the same time,
by studying the rotation of molecules in the droplet, we have an excellent
opportunity to study superfluidity in a finite-size object, opening a new
window on this interesting and a bit misterious phenomenon.
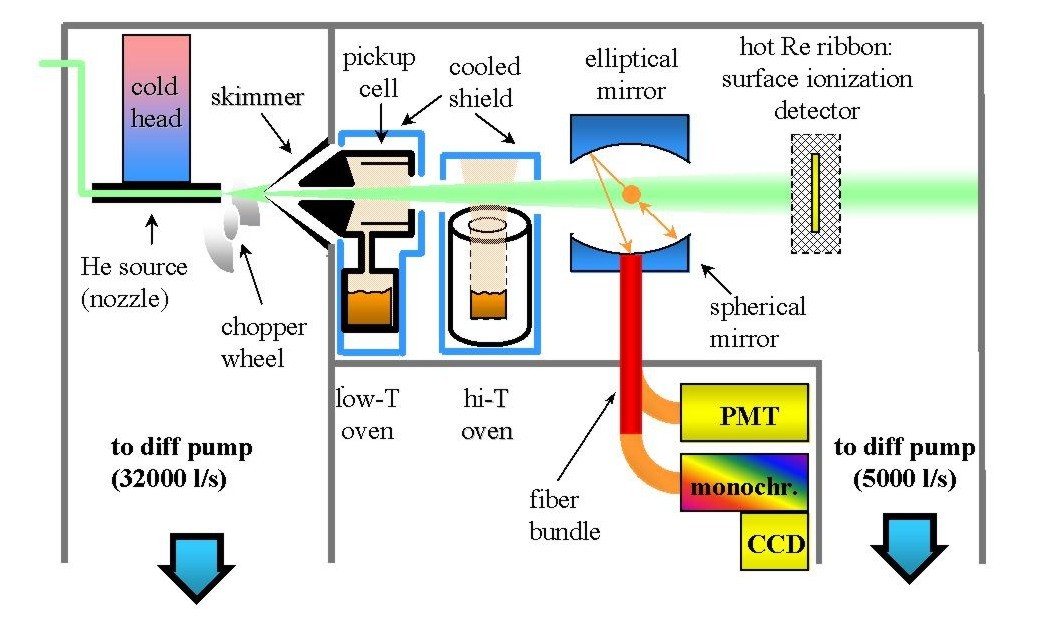
We produce He clusters by expansion in vacuum of high pressure (50-100
atm) He gas through a small orifice, or nozzle, (10 µm diameter) kept
at a temperature of 15-30 K. Under these conditions, a supersonic beam of
He is produced; rapid cooling of the gas occurs in the first few hundred
microns downstream the nozzle, and He atoms aggregate into clusters of a
few thousands molecules each. The clusters fly off into vacuum and cool
by evaporation to the temperature of 0.38 K, where their vapor pressure
becomes negligible.
The supersonic beam has a speed of 400 m/s, so the clusters survive about
a millisecond (the time it takes to travel from the source to the detector).
During this time we (1) load them, (2) excite the doped clusters with our
favorite tunable radiation source, (3) detect their interaction with radiation.
Since 1992, when we detected the first infrared spectrum of a molecule (SF6) in He droplets, our group has carried out a substantial amount of research using HENDI spectroscopy. For sake of simplicity, we will divide the work into two fields: (a) electronic spectroscopy of metal oligomers (1-3 atoms) and (b) rotational/vibrational spectroscopy of molecules.
We have collected the spectra of several electronic transitions of Li,
Na, K, Mg, Al atoms on He droplets. The shifts (wrt the gas phase transitions)
and widths of the spectra tell us about the location of the dopant atom.
Li, Na, K stay on the surface; Mg and Al are fully solvated inside. We have
calculated the He-dopant interaction potentials and we are able to justify
our observations in terms of the energetics of solvation.
We have also observed and characterized Li2, Na2,
K2, NaK, Na3, K3 and found that on helium
droplets, high spin molecules (e.g. triplet Na2 and quartet Na3,
all parallel-spin species) are selectively stabilized over their low spin
counterparts (singlet Na2 and doublet Na3). This
happens because the latter species liberate much more energy upon formation
and destroy the dropletss. Most of the spectra were observed for the
first time, because the high-spin species are either very difficult (dimers)
or next to impossible (trimers) to form in the gas phase. We have also recorded
dispersed fluorescence spectra of most of the above transitions. The spectra
showed that intriguing and unexpected events were taking place, such as
the formation and desorption of Na*He and K*He excimers (never observed
before), and the formation of singlet Na2 and K2 after
excitation of the triplet dimers and the quartet trimers. The latter process
is due to an intersystem crossing from the triplet (quartet) to the singlet
(doublet) manifold. Since a van der Waals bond is converted into a covalent
bond, this can be considered as a prototype laser-initiated chemical reaction.
Finally, we have collected the time-resolved fluorescence arising from some
of those transitions, which allowed us to determine the timescale for the
spin-flip processes mentioned above (... ps).
In the last three years we have built and operated a resonant cavity coupled IR laser spectrometer capable of producing rotationally resolved overtone spectra of molecules containing the CH stretch chromophore, solvated in He droplets. The instrument (which uses bolometric detection) is also capable of producing MW and MW-MW double resonance spectra. We have found that while the molecules are indeed free to rotate in the superfluid medium with very little damping, their moment of inertia is increased by the motion of the liquid around them, by an amount that depends on both the molecule's asymmetry and the strength of its interaction with helium. We have devised (in collaboration with F. Dalfovo) a simple hydrodynamic model which seems to account fairly well for the increase in moment of inertia experienced by the molecules when they become solvated by superfluid liquid helium. In addition, from MW-MW and MW-IR double resonance experiments (the latter in collaboration with R.E. Miller) we have learned about the dynamics of these molecules inside a He droplet, and in particular, we have determined the rotational relaxation timescale: 1-10 ns. This relaxation time is extremely long (× 103) compared to the relaxation in a normal liquid.
We want to switch from chemically stable molecules to radicals: initially
we will just show that radicals can be produced and loaded on helium droplets
(for which we need a clean and relatively cold discharge source). At a later
stage, we will put more than one dopant onto each droplet, selecting species
for which a small (a few units of kT at 0.38 K) barrier to recombination
is believed to exist, and we will initiate the recombination process with
a suitably chosen pulse of radiation.
This will probe the nature of the barrier (via the absorbed photon), as
well as the reaction products (via the emitted photon). A prototype of this
experiment has been already demonstrated with Na3 (quartet) as
the precursor, and Na2(singlet)+Na as the products.
Two important series of experiments will then be tried. In the first we will try to detect high-spin radicals formed by capturing in the droplets two "species", such as two CºN radicals. The stable molecule is of course NºC–CºN, but the dipole-dipole interaction may lead to the NºC···NºC van der Waals complex, even if the spin state of the complex may be favorable to the ricombination. In the second series of experiments we will use a newly acquired powerful uv laser (one of the very first of its kind, to be delivered in October 2000) to dissociate a molecule inside the droplet and study the fragment so formed. Are these going to be trapped by the cluster and eventually recombine? If this is the case, can the fragments' spin be manipulated to hinder the recombination?