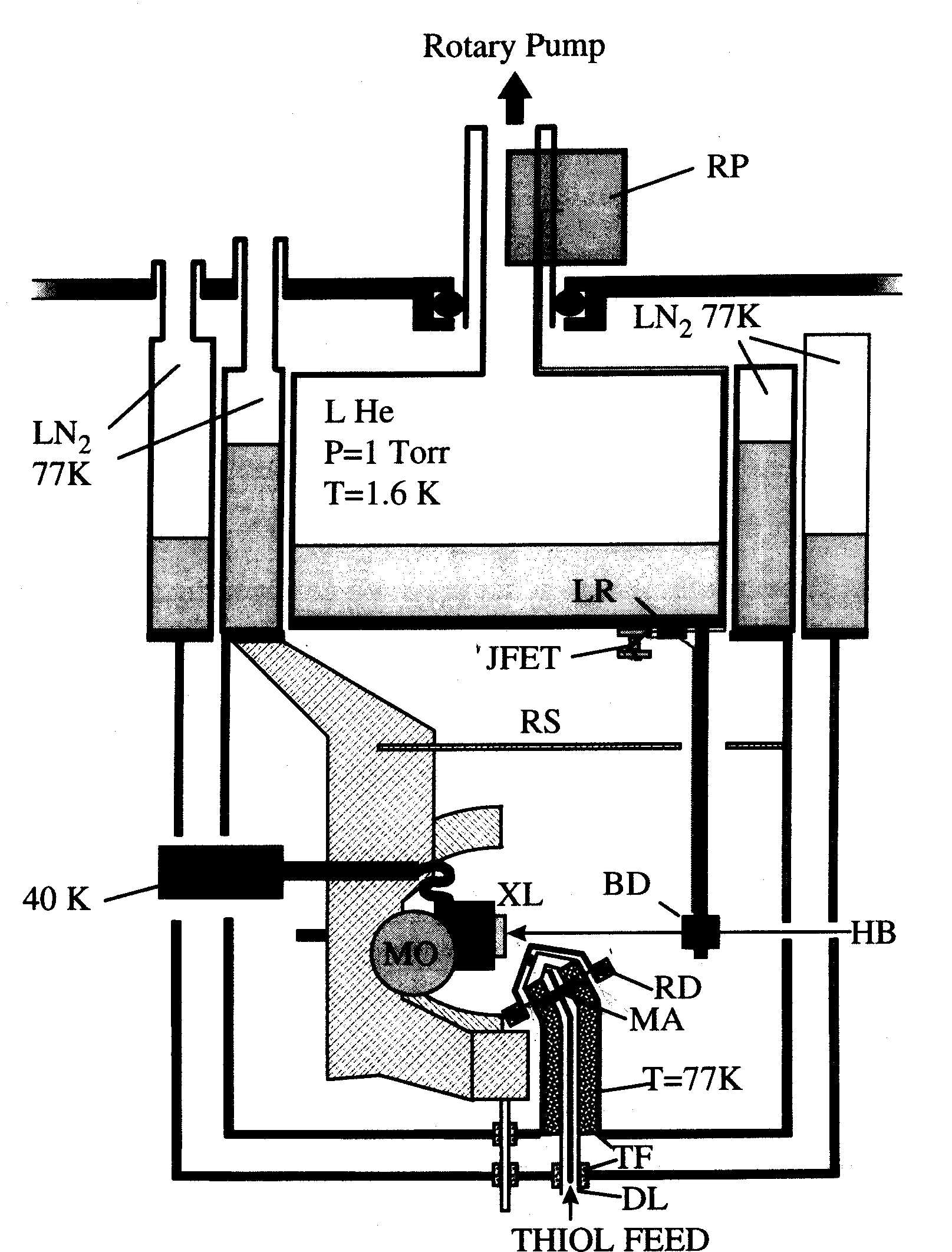
Schematic of the inner part of the
Helium atom diffraction apparatus
(RS: radiation shield, DL: dose line,
HB: incident helium beam, XL: crystal, BD: bolometer detector).
Why?
Self-assembled monolayers (SAMs) of thiol-functionalized molecules on gold have been studied extensively because of their role as model systems to better understand self organization of matter in two dimensions. Their numerous technological applications in corrosion inhibition, nano-fabrication of electronic devices, sensors and non-linear optics are another reason for the considerable attention that these systems have enjoyed during the last 10 years. Although Self-Assembled Monolayers made of long chains of n-alkanethiols (CH3(CH2)n-1SH) on Au(111) have been extensively studied in the past, the driving forces behind the appearance of the (3x2Ö3) super lattice observed at full coverage are still not completely understood. In order to focus on the role played by the sulfur head group minimizing the interactions between chains, we have carried out a He atom diffraction study of the adsorption of the shortest (n=1) thiol radical, obtained by dissociative adsorption of (CH3S)2, on the Au(111) surface.
How?
To characterize the methanethiolate monolayer we used
He atom diffraction. A mono energetic helium beam is produced by supersonic
expansion from a source, which is kept at 70 K. The energy of the He beam
is about 14 meV with a dispersion of ~ 2%.
The beam is collided with the surface at a constant incidence angle around
60o, and the resulting diffraction pattern is detected by rotating
a liquid helium cooled bolometer (kept at 1.6 K by pumping on the liquid
helium) around the surface in a horizontal plane containing the incident
beam and the normal to the surface. The Au(111) surface can be cleaned
under ultra high vacuum through several sputter-anneal cycles (15-30 minutes)
with Ar pressure of 1.3-1.5x10-5 torr and Ar+ energy
of 1.0 keV. The cleanliness of the surface is verified by the observation
of the (0,-1) diffraction and (23xÖ3) reconstruction
peaks of Au(111). Monolayers of dimethyldisulfide were prepared by deposition
from the gas phase through the doser shown in figure 1. Dimethyldisulfide
was purified by several freeze-pump-thaw cycles prior to deposition. By
changing the substrate temperature and the annealing period we optimized
the deposition conditions for obtaining a well resolved overlayer structure.
The thermal stability and the desorption energy of the methanethiolate
SAM was then investigated by changing the annealing parameters and by performing
temperature programmed desorption respectively.

Schematic of the inner part of the
Helium atom diffraction apparatus
(RS: radiation shield, DL: dose line,
HB: incident helium beam, XL: crystal, BD: bolometer detector).
What have we done so far?
We have performed a study of the structural evolution
of methanethiolate on Au(111) surface by Helium atom diffraction. We observed
for the first time that even the shortest thiol forms a (3x2Ö3)
super lattice, which shows that the gold-sulfur interaction plays a major
role in the formation of the super lattice. Our results suggest that at
lower surface coverage the overlayer consists of small domains of (3x2Ö3)
structure. Further dosing enhances surface coverage and, upon annealing
at 290 K, a well-ordered
(3x2Ö3) phase can be
obtained. Formation of the complete (3x2Ö3)
phase in a stepwise manner can be explained by the fact that, without annealing,
it is not possible to achieve saturation coverage. Conversely, we found
that annealing the sample at still higher temperatures (330 K) results
in the transformation of the high density (3x2Ö3)
phase to a lower coverage phase that may be a mixture of (3x2Ö3)
and striped islands. This desorption temperature (which is lower than that
of other thiols) is reasonable in view of the fact that methanethiol monolayers
have smaller interchain interactions than the longer chain thiols. These
results reveal that provided the right deposition conditions and annealing
period for system to reach the equilibrium are being used, there is no
reason to not to obtain the (3x2Ö3) super
lattice, regardless of the chain length of the thiol species. In view of
the disagreement with theoretical predictions of Vargas et al. and
the new possibilities introduced by the work of Molina and Hammer, further
theoretical work is highly desirable both to study the possible gold surface
reconstruction and to analyze quantitatively our diffraction intensities.
Observed chemisorption and physisorption energies were in good agreement
with the literature. Observation of the peaks forbidden by the symmetry
of the previous models indicates a larger distortion in the symmetry of
the unit cell. The large number of diffraction channels caused by large
surface corrugation of the (3x2Ö3) phase
hinders a simple quantitative analysis of the diffraction pattern.
What Next?
As the next step we plan to study the same system by using
grazing incidence X-ray diffraction (GIXD) combined with photoelectron
diffraction (PED) and XPS measurements. In-plane X-ray diffraction will
provide an independent confirmation of the internal symmetry of the (3x2Ö3)
superstructure, while diffraction line-width analysis will give information
on the ordered domain size. Moreover, Qz modulation analysis
around each diffraction peak would provide information on the molecular
form factor, and as a consequence on the orientation of the CH3-S bonds.
The XPS line-shape analysis may be used to individuate, if any, different
kind of sulfur atoms bonded with locally different chemical environments.
Finally, and most importantly, we plan to carry out PED measurements of
the S 2p core level to determine the bonding geometry and the S-Au bond
lengths of the different sulfur atoms in the (3x2Ö3)
unit cell.