|
|
|
|||||||||||||||
Diffusion |
|||||||||||||||||
· Fick's second law describes the
transient situation in which the concentration at a point in the material is
changing with time: |
|||||||||||||||||
 |
|||||||||||||||||
From:
Newey & Weaver, |
|||||||||||||||||
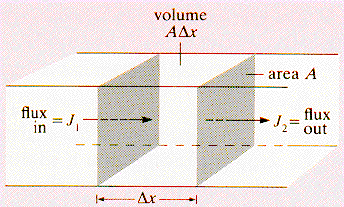
· The net atom increase in the small
region in time Δt is: (J1
- J2) A Δt, and so the rate
of increase in the concentration is: (Δc/Δt) = ((J1 - J2)/
Δx) with J given by Fick's first law. A concentration
independent D(T) gives the form of the second law shown above. |
|||||||||||||||||