|
|||||||||||
|
|||||||||||
 |
|||||||||||
| Carbon
is in group IV A of the periodic table with atomic number 6, an atomic
weight of 12.011, and a density of 2.26. It melts at 3727 C.
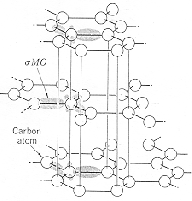
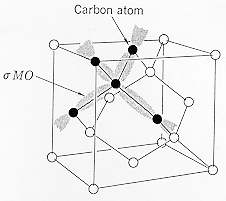
The electronic configuration of C is (1s)2(2s)2(2p)2, and the atomic radius is 0.0914 nm. The stable form of carbon at room temperature is Graphite which has a hexagonal crystal structure. (Top diagram) Diamond is metastable at room temperature and has a diamond cubic crystal structure. (Lower diagram) The diamond cubic structure is a face-centered-cubic lattice with a basis of two carbon atoms. |
|||||||||||
 |
|||||||||||