|
|
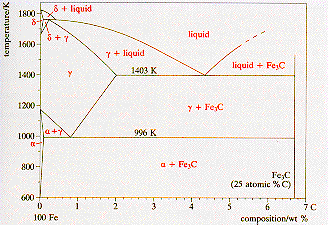
A
carbon steel is an interstitial alloy of only iron and carbon. The carbon
has a limited solid state solubility in the iron, and above 25 atomic %
carbon a compound of iron and carbon, Fe3C
is formed.
The
diagram shows the iron-rich end of the iron-carbon phase diagram. The verttical
line at 6.7 weight % carbon is Fe3C,
and for the field shown this material and iron behave as a binary system.
The equilibrium phases are shown on the diagram. Ferrite (a)
is bcc iron with carbon in interstitial solid solution. Austenite (g)
is fcc iron with interstitial carbon, and d-iron
is bcc iron with interstitial carbon in the high temperature range. Boundaries
with two phase zones represents the solubility limit for carbon in these
phases. |
|
|
|
|
|
|
|
|