|
|
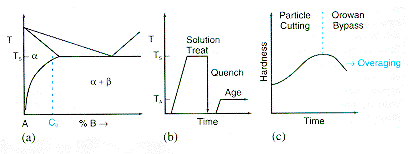
The
diagram below shows three aspects of precipitation hardening. The phase
diagram (a) shows an alloy with a composition C0 in the binary
A-B system. The solubility of B in A decreases rapidly with temperature
as shown by the solidus line separating the a
and the (a
+ b)
phase regions. The material is heated to the temperature T5,
(b) and held at that temperature long enough to produce a homogenious
a-solid solution. Quenching
the material to room temperature keeps B-atoms in solution and produces
a metastable solid solution. This material is taken to a desired degree
of hardness by precipitating the b-phase
in the a-matrix
during a reheating (ageing) process. Diagram (c) shows the hardness as
a function of time for the particular ageing temperature. Other temperatures
will cause the material to come to other precipitation hardened conditions
and hence other hardness values. |
|