|
||||||||
|
||||||||
| Titanium
is a metal in group IVB of the periodic table with atomic number 22, an
atomic weight of 47.90, a density of 4.54 Mg/m3,
and a melting point of 1670 C.
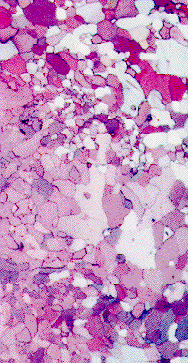
The electronic configuration of Titanium is: (Ar)(3d)2(4s)2, and its atomic radius is 0.147 nm. At room temperature Titanium has the hcp structure (a), it undergoes a polymorphic transition to the bcc structure (b) at 828 C. Alloying elements such as aluminum and vanadium can change the temperature range in which these two forms are stable. The photograph shows an a-b Ti-6Al-4V alloy in which both a and b phases are present at room temperature. The 6 weight % aluminum stabilizes the a phase and the 4 weight % vanadium stabilizes the b phase. E
= 110 GPa, sy
= 500 MPa, UTS = 600 MPa,
|
||||||||
 |
||||||||