Pollution prevention in an organic chemistry research laboratory
EVSC
602-103
Robin Izzo Scott
rmizzo@princeton.edu
December 18, 2000
The issue
This paper will present a number of recommendations to reduce the amount of solvents used and hazardous chemical waste produced in an organic chemistry research laboratory at Princeton University. The focus is on cleaning methods used for glassware and instruments used in compound synthesis, separation and analysis.
background
Princeton University generates more than 30 tons of hazardous chemical wastes each year from all operations, including maintenance, athletics, grounds, utilities and research and teaching laboratories. Roughly two thirds of this waste is generated by research and teaching laboratories.
Hazardous waste generation has decreased significantly over the past five years due to both operational and regulatory changes. In 1996, the New Jersey Department of Environmental Protection significantly changed their hazardous waste regulations, essentially adopting the federal hazardous waste standards. As a result, oils were no longer considered hazardous wastes and were no longer counted toward waste totals. Elemental mercury is also no longer considered a hazardous waste as long as it is sent for reclamation or recycling.
In both cases, the actual use of oil and mercury had not changed and the potential risk to human health and the environment remained the same. Nonetheless, the elimination of oil in particular made it appear that the University had reduced hazardous waste generation by more than seventy-five percent, mainly since oil and oil-contaminated soil from underground storage tank removals no longer counted toward waste totals.
During this same time period, the University's Environmental Health and Safety group began to look for pollution prevention opportunities throughout the campus, with the emphasis on reducing generation of chemical waste. A number of initiatives together helped to reduce hazardous waste production by more than fifty percent and helped to reduce risk to human health and the environment overall by an unmeasured factor. A few of these initiatives are summarized below:
· Replaced most mercury thermometers with non-mercury thermometers.
· Phased out almost all use of chromic acid for cleaning laboratory glassware.
· Reduced the scale of almost all experiments conducted in teaching laboratories.
· Installed chilled water loops fed by the central Chilled Water Plant in most engineering laboratories to replace cooling systems relying on pass-through water cooling.
· Replaced some wet laboratories with computer modeling for Molecular Biology teaching labs.
· Reduced use of ethidium bromide by switching to miniature electrophoresis devices.
· Substituted agar for acrylamide for gel electrophoresis.
· Eliminated almost all 20 liter solvent containers from the Chemistry stockroom, requiring laboratories to purchase smaller containers. This helped reduce the amount of unused chemicals in the hazardous waste stream.
· Improved training program for students, faculty and staff to emphasize pollution prevention and waste minimization in experimental design.
· Instituted clear guidelines as to what can and cannot be disposed via the drain, helping to reduce the amount of hazardous waste entering the sanitary sewer.
· Eliminated the use of ethylene glycol in all HVAC systems, either substituting propylene glycol or moving to a dry system.
· Phased out use of chlorofluorocarbons.
· Replaced hydraulic fluid with vegetable oil in most elevators.
· Phased out most use of oil-based paints.
· Reduced use of pesticides by ninety percent by implementing integrated pest management.
· Replacing spent fluorescent bulbs with low mercury bulbs.
· Placed spill control materials throughout laboratory buildings and at all loading docks. Implemented an improved training program to all affected students and staff.
· Instituted a more comprehensive inspection program that focuses on pollution prevention and waste management.
· Provided secondary containment for all laboratories and maintenance chemicals stored near drains.
All of these measures were intended to be applied throughout campus, in multiple operations. There had not been an attempt to look for pollution prevention opportunities in a single laboratory or single process.
Of all science and engineering departments on campus, the Department of Chemistry uses the largest quantity of chemicals and produces the largest amount of hazardous waste. Each year, this department, as a whole, produces more than nine tons of hazardous chemical waste. Within the department, an organic chemistry research laboratory led by Daniel Kahne is the single largest chemical user and producer of hazardous waste.
Over the past five years, as most other laboratories reduced the amount of waste production, this laboratory group increased the amount produced. This is mainly due to the expansion of the laboratory group itself, which now has more than twenty post-docs, graduate students and undergraduates conducting research.
The majority of the wastes produced are mixtures of organic solvents. Three years ago, the Environmental Health & Safety staff began pouring the five-gallon containers of waste collected in the lab into 55-gallon drums. Looking at the waste manifest and biennial waste reports, it would appear that the amount of waste generated had actually been reduced by approximately thirty percent. However, a closer look reveals that this change is solely due to the fact that more actual liquid will fit into a 55-gallon drum, as opposed to that same drum filled with five-gallon carboys. At most, six five-gallon containers fit into a 55-gallon drum, for a total of approximately 26 gallons of waste.
Given the fact that this one laboratory group produces more than half of all waste generated by the Chemistry Department, the Pareto Principle suggests that pollution prevention efforts beyond those aimed at all laboratory operations be focused here.
analysis
To initiate this pollution prevention opportunity assessment, a variation of the Green Zia approach was utilized. However, at this time, the full assessment is not complete. The steps taken thus far are:
1. Met with representatives to discuss all operations and look broadly at each process to determine where to focus attention.
2. Mapped out the process under review.
3. Analyzed chemical use and waste production for each process step.
4. Conducted root cause analysis.
5. Brainstormed list of options.
None of the options have been tested and the action plan is not complete; however, since this is a real, rather than hypothetical, situation, these steps will be taken over the next few months and continuous measurements and improvements will follow.
The research in this laboratory is focused on carbohydrate synthesis and recognition. The goal is to be able to make carbohydrate libraries using solid-phase synthesis. The laboratory operations generally fall under two main categories: carbohydrate synthesis and biochemical assaying. The biochemical assays do not utilize significant amounts of organic solvents, thus were not included in the assessment.
Almost every laboratory worker is involved in carbohydrate synthesis, each working with a different material, but using similar methods. The types and amounts of organic solvents vary from researcher to researcher, but the following process map is applicable to all of them.
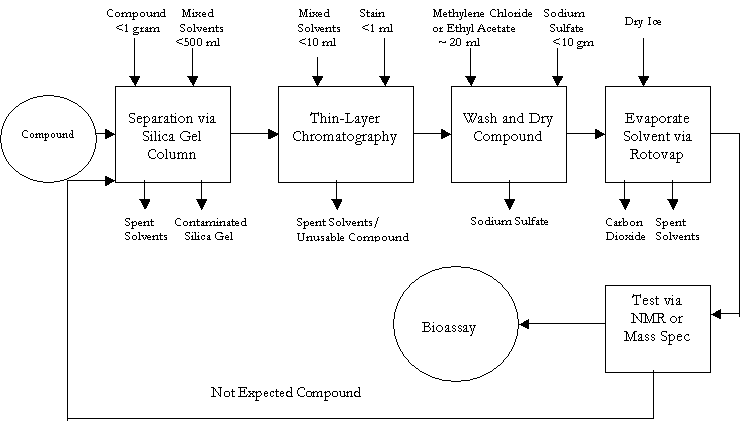
With the exception of the carbon dioxide from the rotovap (which is emitted to air), the spent solvents and other material are disposed as hazardous waste. Energy use from the vacuum system, rotovap, water bath, stirrers and utilities is not included in the process map. Cleaning of glassware, stirrers, spatulas and other laboratory equipment at every phase is also not included in the map.
The amount of silica gel and solvent used for column separation is based on a column packing chart. Depending on the amount of material to be separated, different column sizes and amounts silica gel (measured in centimeters) are specified to achieve a retardation factor of approximately 0.3.
For the column separation and thin-layer chromatography, laboratory workers utilize a mixture of at least two solvents. This mixture differs depending on the compound and is usually determined by trial and error. Since it may differ each time, laboratory workers are unable to continuously reuse the material.
In a given day, each researcher uses approximately two liters of solvents. Each researcher also generates approximately 20 liters of spent solvent waste each month. Considering that the researcher will run the process twice a day and looking at the amount of solvent used in the process, two liters appears to be an excessive estimate.
Cleaning
Where does the other liter come from? What was not considered during the discussion stages, but was observed by the assessor was that seemingly excessive amounts of solvents are used for cleaning glassware, spatulas and other laboratory instruments. When asked how the researcher cleans glassware and instruments, most would say that they first try to clean with Alconox and water and, if that does not work, they then use a small amount of solvent, usually acetone, but often whatever seems to be handy. Indeed, most sinks had small basins of soapy water ready for washing, but they appeared to be used very infrequently.
For example, when preparing to wash and dry the extracted compound, a researcher cleaned the spatula six separate times by opening a waste container, placing a funnel in the mouth of the container and spraying the spatula with at least 15 cc of acetone each time. Each small beaker and Erlenmeyer flask was usually rinsed in the same manner with whatever solvent was available, usually acetone. Even more solvent was used to clean off the permanent marker writing on the glassware. At no time was any type of scrubbing attempted. Rather, the pressure from the steady stream of solvent was expected to clean the glassware.
There was some glassware that was placed in the soapy water for later cleaning. On several occasions, the cleaned glassware would then be rinsed with a solvent to expedite drying. When asked why so much solvent was used for cleaning these instruments, the researchers explained that many of the carbohydrate compounds are difficult to clean without the use of solvents and the brushes supplied to the laboratory are of such poor quality that they do not work very well.
purchasing, storage and transfers
Only ethanol and acetone are purchased in 20 liter containers. All other solvents are purchased in one and five liter containers. It is very rare for the laboratory to have to dispose of unused solvents, unlike most laboratories. The American Chemical Society estimates that 40% of the wastes generated from most laboratories consists of unused chemicals[1]. This particular laboratory does an excellent job of purchasing materials as needed.
Nonetheless, there are scores of liters of flammable and non-flammable solvents stored in the laboratory. All containers are stored in flammable liquid storage cabinets in three main locations. The laboratory spends approximately $10,000 each month on chemical purchase (at least $2000 per month for the most common solvents) and the Environmental Health & Safety office spends approximately $500 each month disposing of waste solvents. In a given month, this laboratory group utilizes at least the following solvents in these approximate amounts:
|
Chemical |
Amount |
Cost |
|
Acetone |
80 liters |
$227.00 |
|
Acetonitrile |
48 liters |
$442.00 |
|
Chloroform |
8 liters |
$42.00 |
|
Ethyl acetate |
48 liters |
$189.00 |
|
Methylene chloride |
120 liters |
$533.00 |
|
Petroleum ether |
64 liters |
$256.00 |
|
Tetrahydrofuran |
16 liters |
$164.00 |
|
Ethanol |
40 liters |
$55.00 |
Laboratory workers did an excellent job keeping containers sealed or otherwise covered to prevent solvent loss from evaporation. Indeed, most laboratory workers not only capped chemical containers when finished, but also placed a layer of parafilm over the cap as an additional barrier.
Vessels used for thin-layer chromatography were also tightly capped at all times to prevent evaporation of the solvents. Flasks and beakers containing solvents were covered with parafilm when not in active use, unless evaporation was desired.
Each work area had a collection of squeeze bottles containing a variety of solvents. Although the squeeze bottles are not capped, the amount of solvent evaporation is likely to be trivial due to the small surface area available. All of these solvents are routinely used.
The 20 liter containers of acetone and ethanol that were in use all had pumps sitting in the container for dispensing. However, in all but one case, the diameter of the pump was significantly smaller than the diameter of the mouth of the container, leaving an approximately two-centimeter space around the pump open. This likely allows for solvent loss through evaporation. For the one exception, a researcher had cut a hole in a plastic cap to tightly fit around the pump.
Other issues
Besides the solvent use, two other rather simple issues were noted. First, most researchers kept the power to the rotovap on at all times, even when not in use. While it is understandable to keep the heating element for the water bath on to maintain temperature, there is no reason to keep the rotary arm portion turned on.
Similarly, water pumps are left running at all times, passing more than a gallon of water into the drain each minute. One problem is that only one closed loop water saver is available for each bench, and many have need for more than one.
None of the thermometers used by this group contain mercury. This lab has been successful in eliminated all sources of mercury without compromise.
pollution prevention opportunities
Pollution prevention opportunities are available for four main categories: cleaning, solvent use during the primary process, storage and water and energy conservation.
cleaning
By far, it appears that the most wasteful solvent use is in cleaning laboratory glassware and equipment. There are a number of alternatives available, four of which were proposed to the laboratory personnel.
The first possibility is purchasing ultrasonicators for each laboratory bench. An ultrasonicator is likely to clean the materials much better than simply soaking them in Alconox and rinsing with water. There are several types of detergents available for use in an ultrasonicator. The most promising may be either Liquinox or Micro 90. Liquinox is a stronger aqueous version of Alconox and, according to the literature, should work well on carbohydrates. Micro 90 is an alternative to chromic acid and has worked well for yeasts and other materials in Princeton University's Molecular Biology laboratories over the past several years.
The cost of a benchtop ultrasonicator is approximately $500, with a monthly cost of approximately $10 for the detergent. If the ultrasonicator is used by two researchers, savings from decreasing use of solvents are an estimated $75 per month, not including disposal costs. The payback period to the laboratory is less than eight months. In addition, this initiative is likely to reduce hazardous waste amounts from this laboratory by approximately forty percent.
Another possibility is the use of an acetone bath. Rather than squirting solvent over the glassware and equipment, one could dip the material into a container of acetone and use an improved brush to aid cleaning. When not in active use, the container should be closed tightly. To further extend the use of this material, one could distill the spent solution and reuse the acetone for the same purpose.
A third alternative is to continue to use the same procedure for cleaning, but use only acetone and collect it separately from the other spent solvents. The spent acetone could be reused for this process until it is unusable. Again, the material could be distilled for reuse. While this is technically reuse, rather than pollution prevention, it is preferable to the status quo. Even if the acetone is only used one extra time, the savings would exceed $20 per month, per researcher.
The last recommended alternative is to purchase better brushes (for a negligible cost) and change the practice to ensure that each material is first cleaned in soapy water before resorting to solvent. For quick drying, every lab bench has a drying oven that is always on and available. If this is still not quick enough, the laboratory could purchase a few extra pieces of glassware and spatulas. The cost of new brushes and extra glassware would be less than $100 per laboratory worker, with a potential savings of $50 per month, with a payback period of less than two months.
main process
Making significant changes to the chromatography process is a bit more difficult to sell to the researchers. In trying to make a carbohydrate library on a solid support, this group is pioneering new technologies. Significant changes could have adverse effects on the yield.
The initial pollution prevention theory was to reduce the scale of the experiment. However, since the carbohydrate synthesis may require multiple reiterations of the process, the researcher does not want to risk not having enough of the material at the start. Since the separation procedure is dependent on the amount to be separated, it is not practical to try to reduce the scale for this step.
Column separation is not the only separation process available to the researchers. For example, recrystallization, distillation or HPLC all could be used for this purpose. However, column separation is much more accurate for this particular application than recrystallization or distillation. HPLC is not only much more time intensive, it also tends to require use of even more solvent. [3].
The researchers did note that one way to reduce use of solvents during the column separation process is to conduct the thin-layer chromatography as the fractions are collected, rather than filling all of the test tubes first and then doing the thin-layer chromatography. By testing the fractions as they are collected, it is likely that the desired compound will be found before all of the samples are collected. Once the compound is found, there is no need to proceed with the column separation, and the additional solvent usually required for the last fractions is not necessary. This can save approximately 250 ml of solvents per separation with no additional cost.
In considering alternatives to thin-layer chromatography, it appears that this method has many advantages over gas chromatography or HPLC. It takes less time, provides the ability to analyze several samples simultaneously and allows flexibility in scheduling each step. From a life-cycle standpoint, it also uses less solvents and very little electricity than the alternatives.
Most compounds are washed with ethyl acetate or methylene chloride. From both a health and environmental standpoint, ethyl acetate poses less risk and is preferred to methylene chloride. Although the solvent is chosen based on performance, most researchers tend to try methylene chloride first. Instead, it is recommended to try using ethyl acetate before resorting to methylene chloride.
The choice of solvents used in thin-layer chromatography and column separation are based on polarity and solvent strength [4], usually chosen by trial and error. Again, the trend is to try methylene chloride first, rather than last. A potential alternative to methylene chloride might be methyl propyl ether [5].
Although technically not a pollution prevention alternative, this laboratory might benefit from distillation and reuse of the solvents used in the column separation phase. The problem noted by the researchers is that the boiling points of the solvents mixed together for the process are so close that distillation and separation is extremely difficult using traditional equipment and methods. The commonly used stills require a difference in boiling point of at least 20°C, and are time and labor intensive. However, over the past few years, distillation technology has improved and there are a number of solvent recovery units available that allow a great deal of flexibility in the range of recoverable solvents and space needed for the equipment. These "digital stills" require less supervision and maintenance and produce a high quality product. Solvent mixtures require a difference in boiling point of at least 5°C, which leaves out a few of the mixtures possible for this laboratory. [6] The cost ranges from $10,000 to $50,000.
storage
All solvents should be stored in tightly closed containers. A simple solution to the problem involving the pumps in the large ethanol and acetone containers is to copy the cap design for the one sealable pump. Since extra plastic caps are plentiful, there is no cost for this proposal.
water and energy conservation
Installation of a second closed loop water system is impractical due to lack of space. Researchers tend to keep the water running because when they keep turning it off and on, the rubber hose sometimes clogs. One could occasionally rinse the hose with a dilute acid or run pressurized air through the hose from time to time to keep it clean. Pressurized air is a better solution from a pollution prevention standpoint since no additional chemicals are needed and it takes a minimal amount of energy.
Laboratory workers are encouraged to turn off the rotary portion of the rotovap when not in use, even if the water bath heater remains on. Since they are on two different switches, this should not be problematic. Laboratory workers might also consider putting the apparatus on an inexpensive (less than $10) timer that would turn the heater on in the morning and turn it off in the evening, or whatever timing suits their regular schedule.
conclusions
The main lessons to be learned from this exercise are that
(1) pollution prevention opportunity assessments can be applied to individual research laboratory operations with little alteration from the industry application, and
(2) the findings are in line with our discussions during this course about typical process applications, such as cleaning and coating, throughout all industries.
For this particular laboratory, the main solvent losses are related to the cleaning process. Most likely, no one intended to clean glassware and laboratory materials in this manner, as one can see from the number of detergent and water baths around the lab. However, the practice of rinsing with solvents likely occurred as a simple timesaving alternative.
The ultrasonicator proposal seems the most likely to make the largest reduction in solvent use. From a life-cycle standpoint, manufacture of the equipment itself is a consideration, particularly since the other suggestions do not introduce new equipment or materials. However, if the solvent savings is truly significant, this alternative should still be on the top of the list.
Solvent recovery is an option that warrants further study. While this is a waste minimization, rather than pollution prevention strategy, the reduction of solvent waste generation and the reduction of the amount of virgin materials purchase may be quite significant. In order for this to be most efficient, it would be best if run centrally for the entire campus, rather than just for one laboratory. Such programs have been very successful at many campuses, including Harvard University and Massachusetts Institute of Technology. Regulatory issues could prevent such a project, particularly if this technology is considered "treatment" according to state and federal hazardous waste regulations, since Princeton University is not permitted for waste treatment.
The researchers in this laboratory mostly utilize safe and effective work practices and appear to be conscientious about chemical use. However, there are plenty of alternatives that they could implement for the sake of pollution prevention and waste minimization. Employment of the techniques described in this report may result in significant cost savings.
The biggest obstacle, however, will be changing the current habits in the laboratory. Over the past several years, laboratory safety and proper waste handling have been receiving more and more attention. Changing the culture of the academic laboratory is difficult, but over time, as more and more new people learn good habits from the start, significant improvements become easier.
The same should be true for pollution prevention habits. Particularly in academia, where we are shaping the safety and environmental culture of scientists of the future, we have an obligation to provide education and instill good work habits in the laboratory. This begins with using microscale techniques at the undergraduate level so that newer scientists begin thinking in smaller scale terms. It continues with encouragement of pollution prevention in the planning phases, such that the person designing a new experiment thinks not only of the technical details, but considers the risk that the proposed chemicals pose to human health and to the environment.
This report also illustrates why one cannot rely simply on regulatory reports to measure waste minimization results. Such reports do not take into account changes in the regulations or changes in how waste is collected or even documented. For this laboratory, it would have appeared that significant improvements had been made in reducing the amount of solvent waste, when the only real change was to the way the waste was packaged.
next steps
For this organic chemistry laboratory, we will choose two or three of the suggested cleaning alternatives and implement them at a few of the lab benches. We will keep track of solvent use for these and other researchers in the group to evaluate the effectiveness of each method.
Alternative chemicals and procedures should also be considered. One way to accomplish this is to have an undergraduate use alternative techniques to synthesize carbohydrates that have already been catalogued. Since all chemistry majors must do a junior and senior thesis, this is a realistic approach.
When we are successful in showing significant cost savings to the laboratory, I will share this information with other laboratories and conduct similar assessments in a few of them. With a bit more experience, I can formalize a technique for completing these assessments and share it with laboratory managers and other interested parties so that the laboratory groups may complete their own assessments. This model for self-assessment has been used successfully for other environmental and safety issues at Princeton University, including hazardous waste management.
REferences and contacts
1.
Laboratory Waste
Minimization and Pollution Prevention: A Guide for Teachers, Battelle
Seattle Research Center, Internet reference http://www.seattle.battelle.org/
services/e&s/P2LabMan/index2.htm, March 1996.
2. The Kahne-Walker Laboratory at Princeton University Department of Chemistry, including all graduate students and post-docs for information regarding thin-layer chromatography and column distillation. Professor Daniel Kahne was most gracious and helpful.
3. Dean, John A. et al, Analytical Chemistry Handbook, McGraw-Hill, New York, 1995
4. Townshend, Alan et al, Encyclopedia of Analytical Science, Academic Press, San Diego, 1995.
5. EPA Green Chemistry Expert System
6. Menzigian, John, "Solvent Recovery: Part I", Campus Compliance Review, Issue 4, December 2000. Also, personal conversation with the author.
7. Personal conversation with Ralph Stuart, Manager of the Environmental Safety Facility at the University of Vermont regarding their pollution prevention focus on laboratory glassware washing. Information about this project is available at http://esf.uvm.edu/labxl/UVM%20XL%20information/UVM%20P2%20Programs/glassware/glassproj.html