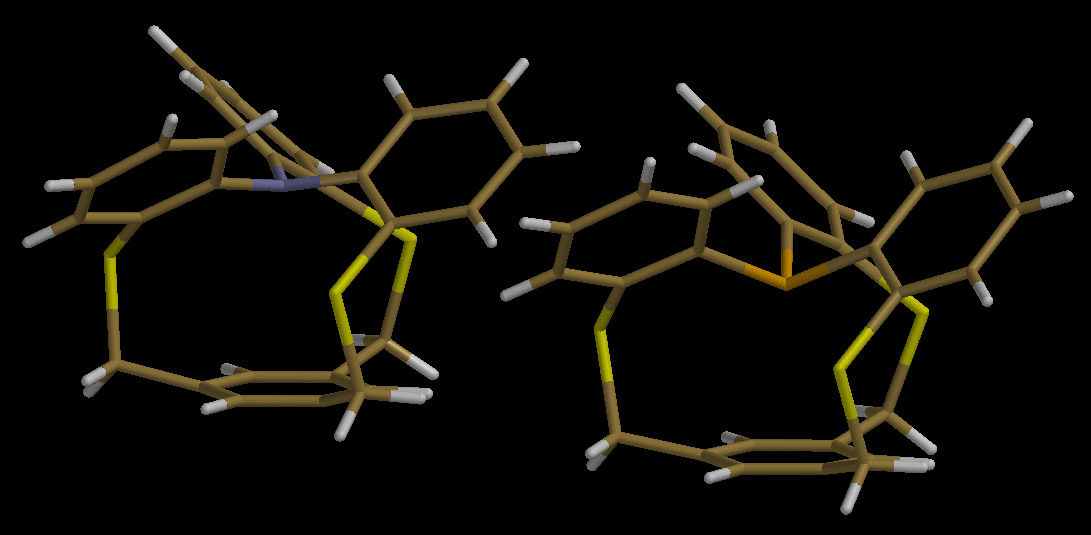
A Barely In-Aminophane
Twenty years ago, we prepared the first in-phosphaphane (below right), a
molecule that still holds the record for the shortest phosphorus-to-arene
contact distance ever reported. Perhaps the most frequently asked question
with regard to this molecule has been, "What about the corresponding amine?"
At last we have successfully synthesized such a triphenylaminophane (it was
not easy!), and its X-ray structure is illustrated below (left). This
amine is barely pyramidalized inward, and its nitrogen atom is not very
close to the aromatic ring, but it is just as unreactive as the phosphaphane.
Interestingly, while the phosphaphane possesses (at least computationally)
distinct in- and out-conformations, the aminophane has only a single,
low-energy conformation, the structure shown. For a brief communication
describing both computational and experimental studies of the in-aminophane,
see Sathyamoorthi, S., J. T. Mague, and R. A. Pascal, Jr., Org. Lett.
2012, 14, 3427-3429.