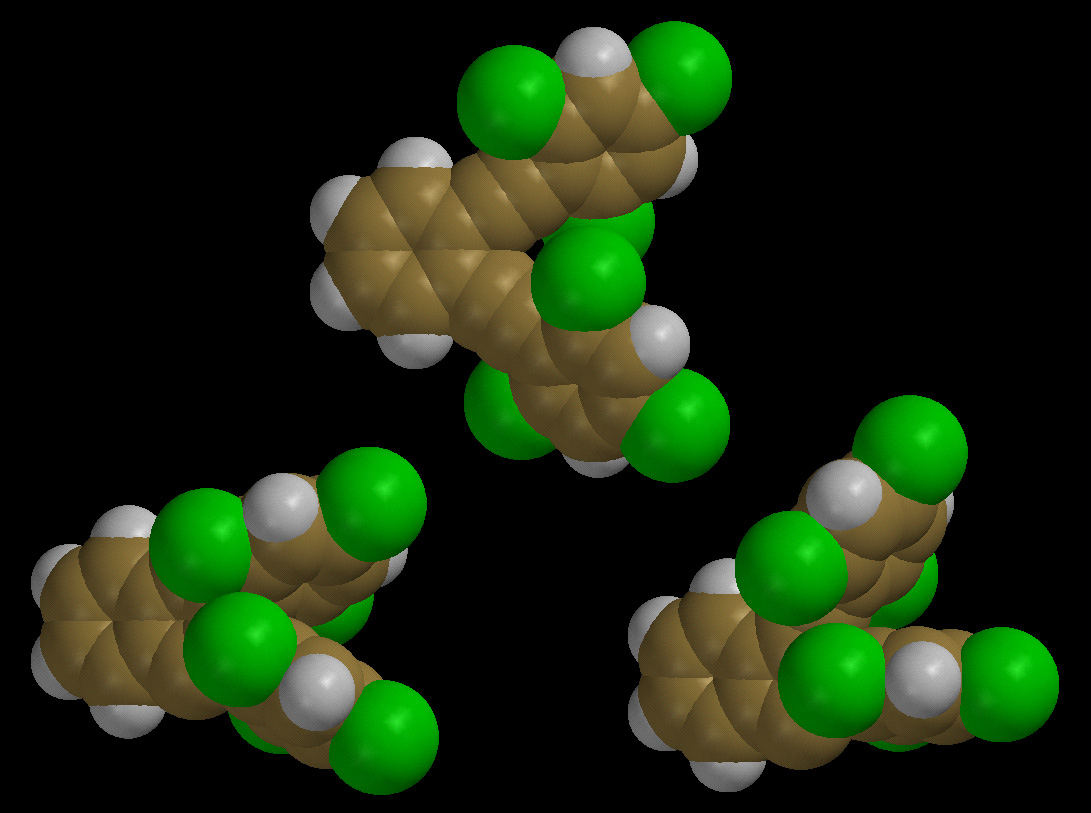
C1-C5 vs. Bergman (C1-C6) Thermal Cyclizations of Enediynes
For bulky diaryl enediynes, a thermal C1-C5 diradical cyclization is
competitive with -- or even preferred to -- the common Bergman (C1-C6)
cyclization. Reduced steric conflict and resonance stabilization of the
incipient C6 radical in the transition state are the principal factors
driving the change in reaction path. Illustrated below are
bis(2,4,6-trichlorophenylethynyl)benzene and the calculated C1-C6 (left)
and C1-C5 (right) transition states for its thermal reactions. Experimentally,
only the C1-C5 cyclization product is observed with this enediyne. For a
not-so-brief communication describing both experimenal and computational
studies of this and related enediyne cyclizations, see Vavilala, C., N. Byrne,
C. M. Kraml, D. M. Ho, and R. A. Pascal, Jr., J. Am. Chem. Soc.
2008, 130, 13549-13551.