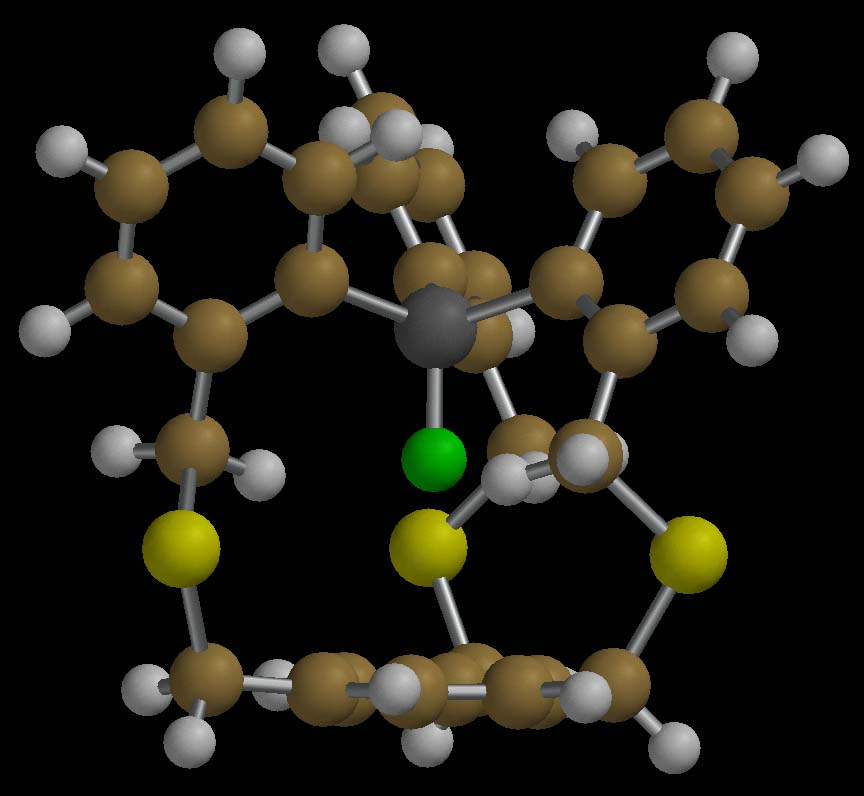
"in-Fluorosilaphane"
The vast majority of the in-isomers of molecules displaying in/out
stereoisomerism possess only hydrogen atoms or lone pair electrons
as bridgehead substituents, because larger inwardly-directed functional
groups are difficult to accommodate in the limited interior space of
such macrocycles. The in-fluorosilaphane below has an in-fluorine atom,
the largest in-functional group in any well-characterized compound. For
a brief communication describing the synthesis and structure of this
molecule, see "An in-Fluorosilaphane: The Largest in-Functionality Is
a Uniquely Encapsulated Fluorine Atom," S. Dell, N. J. Vogelaar, D. M. Ho,
and R. A. Pascal, Jr., J. Am. Chem. Soc. 1998, 120,
6421-6422.