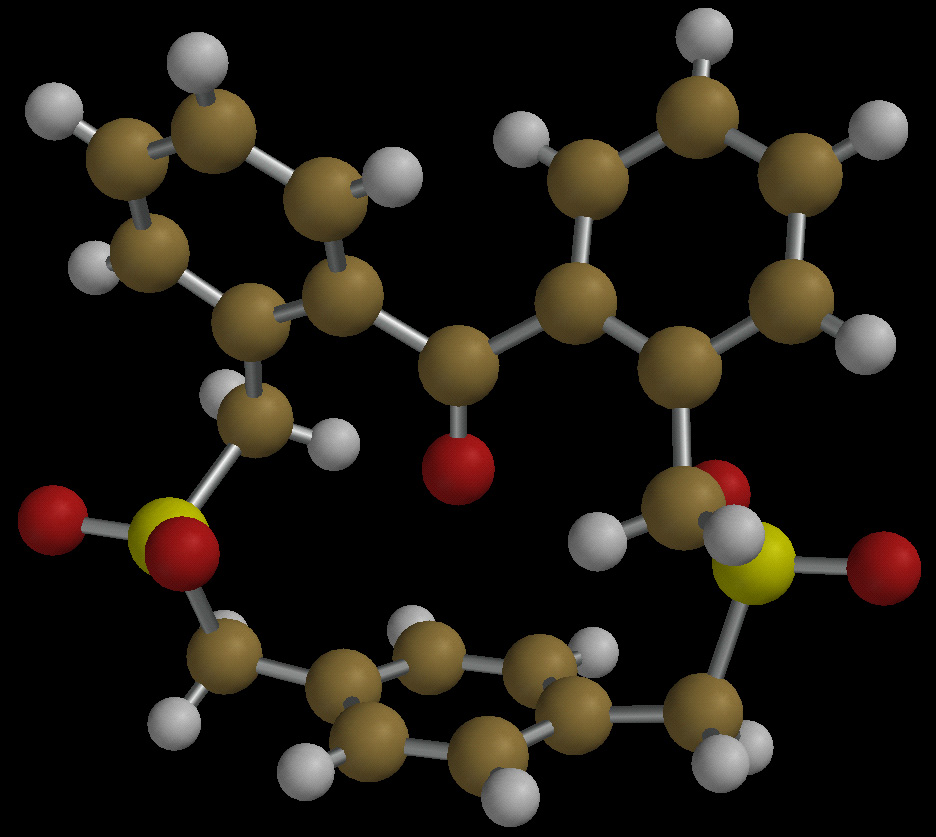
The First In-Ketocyclophane
Over the years, we have prepared many cyclophanes with inwardly-directed
functional groups, but in each case the in-group has had three points of
attachment (three linking arms) to the base of the cyclophane. This
restricts the ability of the functional group to bend away from the base.
The synthesis of an in-ketone presents a special challenge because carbonyl
groups have only two points of attachment, and in most structures can easily
tilt away from the basal benzene ring. However, computational studies
suggested that benzophenone-capped cyclophanes with highly substituted
linking arms would prefer to adopt in-conformations. The X-ray structure
of the first such molecule is shown below. In this bis-sulfone, the ketone
points directly at the aromatic ring, but in its bis-thioether precursor,
which has "floppier" linking arms, the ketone is able to bend away. For
a brief communication describing both computational and experimental studies
of the in-ketocyclophane, see Qin, Q., J. T. Mague, and R. A. Pascal, Jr.,
Org. Lett. 2010, 12, 928-930.