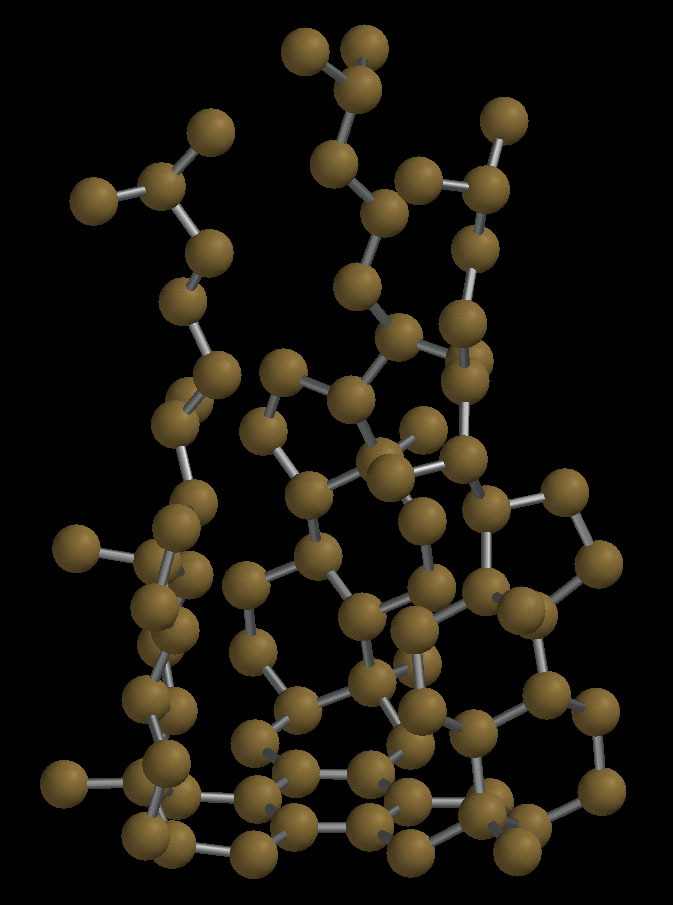
"The Tristeroid"
Trimerization of commercially available coprostanone (a steroid ketone
with a cis A/B ring junction) by treatment with titanium tetrachloride
yields this unusual, chiral, cleft-containing molecule. Only a single
isomer is produced in this triple aldol condensation because the
starting material is enantiomerically pure; a racemic ketone would
give four isomers. The tristeroid was first synthesized by my student
Mary Mathai almost ten years ago, but only in the last year did we
obtain crystals suitable for X-ray analysis. The structure is shown
below, and for a brief communication describing the synthesis and structure
of this molecule see "Trimerization of a Steroid Ketone to Form a Chiral
Molecular Cleft," R. A. Pascal, Jr., M. S. Mathai, X. Shen, and D. M. Ho,
Angew. Chem. Int. Ed. 2001, 40, 4746-4748.