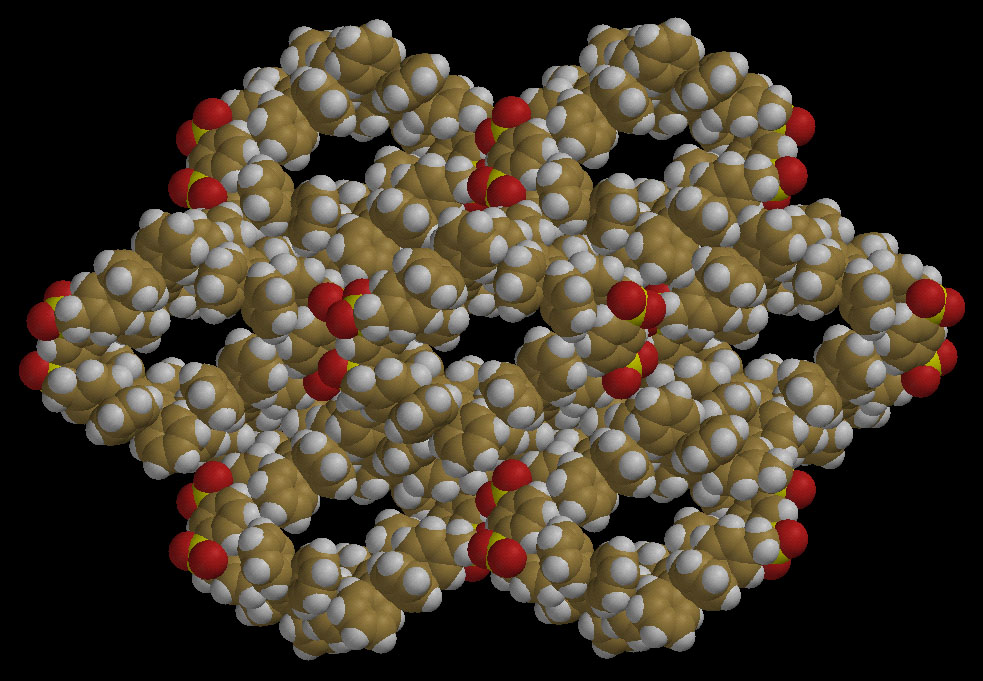
Toward a hydrophobic, "zeolitic", molecular crystal?
This is a view down the b axis of the crystal structure of a giant cyclophane
(MW = 2440) formed by linking two 1,3-bis(pentaphenylphenyl)benzene subunits
together. The molecule is sufficiently rigid that the central cavity
cannot collapse, and thus it forms a parallelogram with each side a
polyphenylbenzene group. The cyclophane packs in the crystal so that each of
its four polyphenylbenzenes is stacked against a similar group in an adjacent
molecule, with the result that long, wide channels are present in the
crystal. Each of these channels is wide enough to accommodate two or three
solvent molecules side by side (the crystal contains disordered ether and
DMSO). Although some of our polyphenyl aromatics form robust, thermally
stable (to >400 deg C) structures with similar (but narrower) channels, loss
of the interstitial solvent causes these crystals to decompose.
A paper describing the syntheses and structures of this and related giant
cyclophanes appeared in the November 17 issue of The Journal of Organic
Chemistry ("Giant Cyclophanes Built from Polyphenyl Aromatic Substructures,"
R. A. Pascal, Jr., L. Barnett, X. Qiao, and D. M. Ho, J. Org. Chem.
2000,
65, 7711-7717), and this molecule was featured on the cover of that issue!