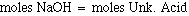 (eqn.
8)
(eqn.
8)Unknown Acid:
The unknown acid has one replacable hydrogen, and at the endpont the stoichiometric relationship between NaOH and the Unk. Acid is 1:1. At the endpoint then:
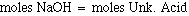 (eqn.
8)
(eqn.
8)
The molcular weight is the number of grams per mole. Since we know how many grams of Unknown Acid we started with, then the molecualr weight can be calculated by:
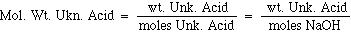 (eqn.
9)
(eqn.
9)
 (eqn.
10)
(eqn.
10)
where vol. NaOH is the volume of NaOH solution used in titration of the Unknown Acvid. As before the volume of NaOH must be converted to liters. There is no uncertainity analysis required for the Unknow Acid. You should go to the window to check your value of the Unknown Acid. When your value for the molecular weight of the Unknown Acid agrees with the accepted value then you have accurately determine the molecular weight. Accuracy is the agreement of a particular value with the accepted value.