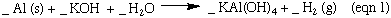
Chemistry:
The synthesis of Kalinite starting from Al metal is outlined in the four equations below. N.B These reactions are not balanced. You need to balance these reactions for the lab report.
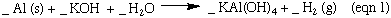
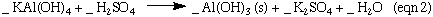
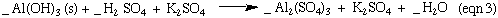

Aluminum is an example of an amphoteric metal. It is soluble in both an acidic and basic solution. Most metals, iron for example, are only soluble in acidic solution and form percipitates in basic solutions. In equation 1 the aliminate ion, Al(OH)4- , is the soluble form of aluminum in basic solutions. Treatment of Al(OH)4- the with acid first forms Al(OH)3 as a percipitate. This percipitate dissolves when more acid is added. You may have noticed that K2SO4 is included here in equation 3 , but was not included in equation 3 in the lab manual. The K2SO4 is a spectator ion, and is it not involved in the reaction. But since the Al(OH)3 formed in equation 3 was not isolated, the K2SO4 ions are still there in the solution.