 (eqn
5)
(eqn
5)
% yield Calculation:
The % yield is a measure of the efficiency of a chemical reaction and is defined as:
 (eqn
5)
(eqn
5)
where the actual yield is the number of grams of product obtained in the reaction. The theoretical yield is the maximum number of grams of product that can be formed in the reaction. To calculate the theoretical yield first determine which reactant is the limiting reagent. In this synthesis, the Al metal is the limiting reagent. All the other reagents, KOH, H2SO4, were added in large excess. Next determine the stoichiometric relationship between the limiting reagent and the product. Overall for the conversion of Al metal to KAl(SO4)2•12 H2O
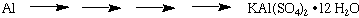 (eqn
6)
(eqn
6)
we see that the stoichiometric relationship for Al: KAl(SO4)2•12 H2O is 1:1. To calculate the theorectical yield for KAl(SO4)2•12 H2O then
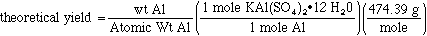 (eqn
7)
(eqn
7)
where 474.39 g/mol is the molecular weight of KAl(SO4)2•12 H2O. Now the % yield can be calculated using eqn 5.
Remember to answer the dicussion questions on page 17 of the lab manual.