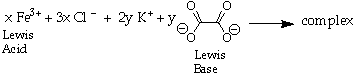
In this experiment you synthesized an iron complex from the reaction of ferric chloride (FeCl3) with potassium oxalate (K2C2O4). This reaction is shown below.
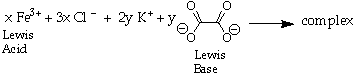
In this reaction the ferric ion (Fe3+) is acting as a Lewis Acid and the oxalate anion (C2O42-) as a Lewis Base. A Lewis Acid is an electron pair acceptor and a Lewis Base is an electron pair donor. The oxalate anion is donating electrons to the vacant 3d and 4s orbitals on iron. The potassium and chloride ions are spectator ions. (see your textbook for a discussion of Lewis Acids and Bases.)
From a series of qualitative tests and a microanalysis of this complex formed, you will demonstrate some of the properties of this complex and suggest a charge balanced formula for the complex.