You performed two different titrations in this part of the experiment. First you titrated the Unknown Acid, and then sulfamic acid, H2NSO3H, to standardize your NaOH solution. We will discuss the standardization titration first.
Titration of Sulfamic Acid (HSA) with NaOH:
Sulfamic Acid is a strong acid (pKa =1.0) and completely dissociates in an aqueous solution.
![]() (eqn 11)
(eqn 11)
The pH at the equivalence point is determined by the dissociation of water. At 25 °C, the pH is 7.00. An example titration curve is shown below.

At the equivalence point then:
![]() and
and ![]() (eqn 12)
(eqn 12)
Rearrangement of this equation to solve for MNaOH gives
![]() (eqn 13)
(eqn 13)
where Mol. Wt. HSA = 97.09 g/mol, and vol. NaOH is the volume of NaOH at the equivalence point expressed in Liters. In the above example this value is 50.00 mL.
Draw a titration curve for the titration of Sulfamic Acid with NaOH. From your curve determine the vol. of NaOH required to reach the equivalence point, and calculate the MNaOH using eqn 13.
Titration of Unknown Acid with NaOH:
Consider the following titration curve:
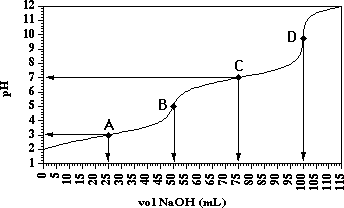
Examination of this titration curve we see four points of interest, labeled A,B,C, and D. There are two flat buffer regions, A and C, and two rapidly rising equivalence point regions, B and D. This titration curve is indicative of a diprotic acid (H2A). Each hydrogen is neutralized stepwise and a Ka expression can be written for each neutralization step.
![]()
![]() (eqn 14)
(eqn 14)
![]()
![]() (eqn 15)
(eqn 15)
Equivalence Points; Points B and D.
Point B is the first equivalence point. The first replaceable hydrogen has been completely removed and the major chemical species is HA-. At point B:
moles H2A = moles HA- = moles of NaOH (eqn 16)
and
![]() and
and ![]() (eqn 17)
(eqn 17)
Combining eqns 16 and 17, and rearranging to solve for Mol. Wt. H2A we get
![]() (eqn 18)
(eqn 18)
where wt. H2A is the weight of unknown acid you started the with, MNaOH is the concentration you determined from the sulfamic acid titration, and vol. NaOH is the volume of NaOH at the first equivalence point expressed in Liters. In the above example, this value is 50.00 mL.
Point D is the second equivalence point. Now the second replaceable hydrogen has been completely removed and the major chemical species is A2-. At point D:
moles H2A = moles A2- = 0.5* moles of NaOH (eqn 19)
and
![]() and
and ![]() (eqn 20)
(eqn 20)
Combining eqns 18 and 19, and rearranging to solve for Mol. Wt. H2A we get
![]() (eqn 21)
(eqn 21)
where now the vol. NaOH is the volume of NaOH at the second equivalence point expressed in Liters. In the our example this value is 100.00 mL. Note that vol. NaOH at Point B = 0.5 vol NaOH at Point D.
Buffer Regions; Points A and C.
The buffer regions are characterizes by a small change in pH with a large change in NaOH added. Point A is the middle of the first buffer region. The chemistry involves the first dissociation of H2A
![]() (eqn 14)
(eqn 14)
At Point A the [H2A] = [HA-], and the Ka1 expression simplifies to
![]() (eqn 22)
(eqn 22)
Now take -log of both sides of eqn 22 and we get
![]() (eqn 23)
(eqn 23)
For this example the value of pKa1 = 3.0. Also note that the vol. NaOH at Point A = 0.5*vol. NaOH at the first equivalence point (Point B).
Point C is the middle of the second buffer region. The chemistry involves the second dissociation step of H2A
![]() (eqn 15)
(eqn 15)
At Point C the [HA-] = [A2-], and the Ka2 expression simplifies to
![]() (eqn 24)
(eqn 24)
Now take -log of both sides of eqn 24 and we get
![]() (eqn 25)
(eqn 25)
For this example the vol. NaOH at Point C = 0.75*vol NaOH at the second equivalence point.
Construct a titration curve for the titration of your Unknown Acid with NaOH, and:
Remember to answer the discussion questions.