The crystals of sodium thiosulfate (Na2S2O3•5 H2O) that you weigh out are hydrated, and have a tendency to lose their water of crystallization. Therefore a exact concentration of Na2S2O3 can not be determined by weighing the solid and dissolving in a volumetric flask. However, the exact concentration of a Na2S2O3 solution can be readily determine by titration with a primary standard. The primary standard used in this experiment is potassium iodate (KIO3). The exact concentration of KIO3 can be determined from dissolving an exact weight of KIO3 in a volumetric flask.
The reactions involved in this titration are:
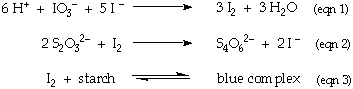
Starch is added late in the titration since at high concentrations of I2 the starch - I2 reaction (eqn 3) is not reversible.