 SCIENCE/TECHNOLOGY
SCIENCE/TECHNOLOGYVolume 77, Number 23
CENEAR 77 23 pp.
ISSN 0009-2347
C&EN Washington
A skeleton, an abalone shell, an air intake cover from a Toyota Cam ry, and a bathroom deodorizer used in Japan might not seem to have much in common--but they are all based on nanocomposites.
Nature has been producing remarkable nanocomposites like bone and nacre (the pearly, iridescent inner layer of the abalone shell) for millions of years. In recent decades, scientists have slowly been learning how to prepare nanocomposites for a variety of applications, including structural materials, high-performance coatings, catalysts, electronics, photonics, and magnetic and biomedical materials. With the exploding interest in "all things nano" in the 1990s, the pace of nanocomposite R&D has picked up. Commercial products based on these materials, such as the aforementioned auto part and deodorizer, are emerging with greater frequency. Many nanocomposite-based products are reportedly heading for commercialization in the next few years.
Behind the push for nanocomposites is a singular fact: They offer useful new properties compared to conventional materials, says professor Richard W. Siegel, head of the materials science and engineering department at Rensselaer Polytechnic Institute (RPI) in Troy, N.Y. When two or more phases are mixed together to make a composite, one can often obtain a combination of properties that are not available in any of the individual components.
Sidebar: Nanoscale materials special issue
What makes a nanocomposite especially interesting is that at least one of its phases has one or more dimensions--length, width, or thickness--in the nanometer size range, usually defined as 1 to 100 nm. This is the range "where phenomena associated with atomic and molecular interactions strongly influence the macroscopic properties of materials," according to Ilhan A. Aksay, a professor in the chemical engineering department at Princeton University. But this is also the length scale where our knowledge of how to synthesize and process designed materials is weakest, he adds.
Nevertheless, scientists know that the catalytic, mechanical, electronic, optical, and other properties of a material are significantly and favorably altered when that material is fashioned from nanoscale building blocks. Siegel points out, for instance, that nanocrystalline copper is up to five times harder than conventional micrometer-sized copper. And ceramics, which normally are brittle, can be made more easily deformable if their grain size is reduced to the low nanometer range. Such improved properties can also be produced in nanocomposites, in which the building blocks--say, nanoscale metal particles or nanometer-thick filaments or sheets of a ceramic--are dispersed in a matrix of another material, such as a polymer.
As Siegel notes in a report on nanostructure science and technology that was recently posted on the web (http://itri.loyola.edu/nano/final/), "Every property has a critical length scale, and if a nanoscale building block is made smaller than that critical length scale, the fundamental physics of that property starts to change."
An example of this, he tells C&EN, is light scattering. As the particle size shrinks to a fraction of the wavelength of light, the particles won't scatter light of that wavelength anymore. Thus, if the particles are made small enough, they can be used in a composite that will be transparent to light. This is the principle behind sunscreen lotions that contain nanoparticles. The particles are sized to scatter ultraviolet light, but not visible light. So when slathered on the skin, the sunscreen is transparent to the eye but blocks the sun's harmful UV rays.
Because the building blocks of a nanocomposite are nanoscale, they have an enormous surface area, and therefore there are a lot of interfaces between the two intermixed phases, says Pulickel M. Ajayan, a professor of materials science and engineering at RPI. The special properties of the nanocomposite arise from the interactions of its phases at the interfaces. By contrast, in a conventional composite based on micrometer-sized fillers such as carbon fibers, the interfaces between the filler and matrix constitute a much smaller volume fraction of the bulk material and therefore influence its properties to a much smaller extent, Ajayan explains. Phases interacting on the nanometer scale can "bring in new physics," he says, and hence new properties.

Lately, Siegel points out, there has been "renewed interest" in nanocomposites because researchers have been expanding the range of available nanoscale building blocks, and with that, their ability to build new architectures. And new architectures can lead to new properties, which further fan interest.
Many of these new architectures and properties were showcased at "Nanocomposite Materials: Design & Applications," a conference held March 28 to April 2 at a snow-enveloped ski resort near Anchorage, Alaska. The conference was held under the auspices of the New York City-based United Engineering Foundation and was chaired by Siegel and Ajayan.
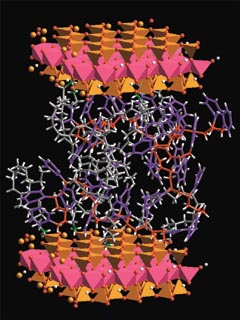
One theme that ran through the conference was how nature has been a source of inspiration for many researchers seeking to design useful nanocomposites. One of those researchers is C. Jeffrey Brinker, a materials scientist at Sandia National Laboratories and the University of New Mexico Center for Microengineered Materials, both in Albuquerque. Although Brinker was not at the conference, he and his coworkers published an article last month [Adv. Mater., 11, 579 (1999)] that stylishly echoed one of the key points made at the Alaska meeting: "The mollusk shell," they wrote, "has long been heralded as the Holy Grail of natural materials design and construction. Its microlaminated architecture, composed of alternating layers of aragonite (a crystalline form of calcium carbonate) and a rubbery biopolymer, has evolved over millions of years to simultaneously provide strength, hardness, and toughness to a lightweight material." Brinker and coworkers went on to note, for example, that abalone nacre composed of only 1% by weight of polymer is twice as hard, and 1,000 times tougher, than its constituent phases.
Bone is another natural nanocomposite with impressive toughness and strength. It consists in large part of nanoscale, platelike crystals of hydroxyapatite, Ca10(PO4)6(OH)2, dispersed in a matrix of collagen fibers. Hydroxyapatite and collagen by themselves are not particularly promising structur-al materials. But when these and other ingredients are assembled to form the complex microstructure of bone, the resulting nanocomposite offers properties that have proven extremely difficult to match with synthetic materials.
As Paul V. Braun, an assistant professor of materials science and engineering at the University of Illinois, Urbana-Champaign, observed at the conference, "Nature has a control of symmetry and dimension that we have yet to achieve."
Although nature is a tough act to follow, many research groups seeking to develop artificial bone or bone implant materials have chosen to start with synthetic versions of nature's own material--hydroxyapatite--in large part because it is so similar to bone mineral and therefore would be compatible with bone and biological tissue. Synthetic hydroxyapatite is already widely used in orthopedic and dental implants, but its applications have been limited by its poor mechanical properties and difficulties in its processing. For one thing, conventionally prepared hydroxyapatite has not been found to have the strength and fracture-resistance necessary for use in load-bearing implants, according to Edward S. Ahn, a graduate student working in the laboratory of chemical engineering professor Jackie Y. Ying at Massachusetts Institute of Technology.

At the Alaska conference, Ahn presented results demonstrating that the mechanical properties and processing of hydroxyapatite can be significantly improved if it is synthesized as a nanostructured material. Ahn and his MIT coworkers, including former undergraduate student Nathaniel J. Gleason, start by precipitating hydroxyapatite from a solution containing calcium nitrate and diammonium hydrogen phosphate, (NH4)2HPO4. The precipitate must then be aged, washed, ground, dried, calcined, and sintered to obtain the final, dense ceramic body. Ahn tried out many different synthetic and processing conditions until he settled on the combination that gave material with the best behavior.

Conventionally synthesized hydroxyapatite can decompose or pose other
difficulties during sintering (the high-temperature process in which
ceramic grains coalesce into a dense body having little, if any,
porosity). But according to Ahn, the nanostructured hydroxyapatite (NHAP)
he prepared can be sintered more easily and at a lower temperature than
ordinary hydroxyapatite. Moreover, NHAP is stable to a much higher
temperature (1,300 ![]() C)
than is the conventional ceramic.
C)
than is the conventional ceramic.
Because of its uniform and ultrafine structure, NHAP is harder to bend or compress than both the conventional ceramic and dense bone itself, Ahn said. This suggests that NHAP might be strong enough to use in a load-bearing implant. Nevertheless, although NHAP is more fracture-resistant than conventional hydroxyapatite, it is not as tough as dense bone.
To remedy that, the MIT researchers added a small amount of partially stabilized zirconia (ZrO2 containing 3 mole % Y2O3) to the NHAP during the initial precipitation step. Zirconia is often used to mechanically reinforce ceramic matrices against cracking. In the past, though, researchers who had used large amounts of zirconia to reinforce hydroxyapatite had encountered difficulties in the high-temperature processing of their nanocomposites. Ying's group was able to avoid those problems by using a colloidal addition technique. This technique enabled the group to achieve a more uniform, ultrafine dispersion of zirconia. This, in turn, allowed the zirconia grains to interact with the NHAP matrix to a greater degree, leading to a uniformly tougher material.
As Ahn reported in Alaska, the nanocomposite with slightly less than 3% by weight of zirconia was found to be harder and more fracture-resistant than either NHAP alone or nanocomposites with a much higher loading of ZrO2. In fact, the fracture toughness of this nanocomposite is the same as that reported for the least fracture-resistant dense bone. "It puts us in the ballpark," said Ahn, who is exploring ways to further improve the material's toughness. The challenge will be to do that while still keeping the zirconia level low, thus ensuring that the nanocomposite remains biocompatible.
This approach of first creating a nanostructured phase and then tweaking its properties by adding a finely dispersed secondary phase also has been used by Ying's group to make advances in the area of high-temperature catalysis. Ying is interested in developing catalytic materials with very high surface area that will remain highly active and stable at very high temperatures. This is a major challenge, she explained at the Alaska meeting, because at high temperatures sintering occurs more readily. Sintering reduces the surface area of the material, degrading its catalytic activity.
Catalysts that can tolerate very high temperatures are needed for the
catalytic combustion of natural gas, which consists largely of methane
and other light hydrocarbons. Many industries are interested in burning
natural gas for energy because it is a cleaner fuel. Compared with coal
and higher hydrocarbons, natural gas combustion releases significantly
less carbon dioxide per unit of energy released, Ying said. But to
achieve efficient burning, the methane flame must be maintained at a
very high temperature--more than 1,400 ![]() C.
Unfortunately, combustion at this temperature causes nitrogen and oxygen
in the air to react to form nitrogen oxides (NOx), which
contribute to acid rain and smog formation.
C.
Unfortunately, combustion at this temperature causes nitrogen and oxygen
in the air to react to form nitrogen oxides (NOx), which
contribute to acid rain and smog formation.
If the combustion of methane could be made catalytic, Ying pointed
out, a stable flame could be sustained at a lower temperature and NOx
emissions could be substantially reduced. But the catalyst would have to
be stable to 1,300 ![]() C.
That's a tall order, she noted, since most chemical processes require
catalysts to function only in the range of 300 to 700
C.
That's a tall order, she noted, since most chemical processes require
catalysts to function only in the range of 300 to 700 ![]() C.
C.
The most common oxidation catalysts, based on noble metals or base
metal oxides, can't be used above 800 ![]() C,
according to Ying. Complex oxides, such as barium hexaaluminate, BaO
C,
according to Ying. Complex oxides, such as barium hexaaluminate, BaO![]() 6Al2O3
(BHA), are more promising because they retain their activity at much
higher temperatures. But the methods conventionally used to make this
compound require it to be crystallized at 1,300
6Al2O3
(BHA), are more promising because they retain their activity at much
higher temperatures. But the methods conventionally used to make this
compound require it to be crystallized at 1,300 ![]() C,
which reduces the surface area and hence its activity.
C,
which reduces the surface area and hence its activity.
Another problem is that conventional BHA has a relatively high
"light-off temperature" of about 710 ![]() C.
This is the temperature at which methane conversion "kicks
off" or begins to be significant--specifically, when 10% of the
methane in the stream is combusted. Ideally, Ying pointed out, you want
the combustion to kick off by about 400
C.
This is the temperature at which methane conversion "kicks
off" or begins to be significant--specifically, when 10% of the
methane in the stream is combusted. Ideally, Ying pointed out, you want
the combustion to kick off by about 400 ![]() C.
C.
To make a catalyst that would work from 400 to 1,300 ![]() C,
Ying knew that she and her coworker on the project, graduate student
Andrey J. Zarur, would need to find a way to prepare nanoscale
crystallites of BHA at low temperatures. The problem with the
conventional wet methods used to prepare BHA is that they involve the
hydrolysis of a mixture of two precursors such as a barium alkoxide and
an aluminum alkoxide. Since these precursors have very different
hydrolysis rates, the product usually consists of a mixture of barium
oxide and aluminum oxide phases. To get these phases to form BHA, they
must be heated to 1,300
C,
Ying knew that she and her coworker on the project, graduate student
Andrey J. Zarur, would need to find a way to prepare nanoscale
crystallites of BHA at low temperatures. The problem with the
conventional wet methods used to prepare BHA is that they involve the
hydrolysis of a mixture of two precursors such as a barium alkoxide and
an aluminum alkoxide. Since these precursors have very different
hydrolysis rates, the product usually consists of a mixture of barium
oxide and aluminum oxide phases. To get these phases to form BHA, they
must be heated to 1,300 ![]() C,
but that produces BHA with low surface area and poor catalytic activity.
C,
but that produces BHA with low surface area and poor catalytic activity.
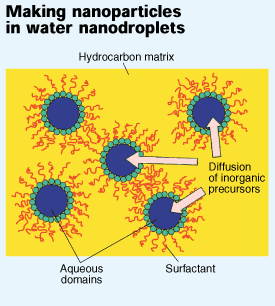
To get around this problem, Ying and Zarur developed a synthesis that is carried out in a reverse emulsion of 15 to 20% water (by weight) in isooctane. This relatively high water content is made possible by the mixture of surfactants they use, which also allows them to stabilize microemulsions with water droplets as small as 5 nm across. Since barium and aluminum alkoxides react only in the presence of water, the two precursors must diffuse through the isooctane and into the water droplets, which serve as nanoreactors. And since the two alkoxides diffuse through isooctane at similar rates, they react to give a homogeneous BHA product. Moreover, the size of the BHA particles is limited by the nanometer-sized pockets of water in which they are produced, Ying said.
Unlike conventional BHA, which must be crystallized at around 1,300 ![]() C,
the BHA nanoparticles prepared in Ying's lab can be crystallized at
1,050
C,
the BHA nanoparticles prepared in Ying's lab can be crystallized at
1,050 ![]() C
because they are much more chemically homogeneous.
C
because they are much more chemically homogeneous.
Even when these BHA nanocrystals are exposed to temperatures as high
as 1,300 ![]() C,
further grain growth is minimal--the final grain size is 30 nm--and the
surface area, which is at least 100 square meters per gram, remains
considerably greater than the 15 sq m per g determined for
conventionally produced BHA, Ying told conference attendees.
C,
further grain growth is minimal--the final grain size is 30 nm--and the
surface area, which is at least 100 square meters per gram, remains
considerably greater than the 15 sq m per g determined for
conventionally produced BHA, Ying told conference attendees.
When the nanostructured BHA was tested in methane conversion under
industrially realistic and challenging conditions, Ying reported, it
sustained "very good activity all the way through 1,300 ![]() C,
even in the presence of lots of water"--conditions that kill many
other catalysts. The nanophase catalyst also achieved a light-off
temperature of 590
C,
even in the presence of lots of water"--conditions that kill many
other catalysts. The nanophase catalyst also achieved a light-off
temperature of 590 ![]() C--a
decrease of 120
C--a
decrease of 120 ![]() C
compared to the conventional material.
C
compared to the conventional material.
But that was not good enough for Ying. She had 400 ![]() C
in her sights because noble-metal catalysts kick off in that
vicinity--although they don't survive at the higher temperatures that
BHA can tolerate.
C
in her sights because noble-metal catalysts kick off in that
vicinity--although they don't survive at the higher temperatures that
BHA can tolerate.
The key to further progress lay in finding a second phase to mix in
with the BHA. Zarur and Ying tried a number of materials, finally
settling on cerium oxide (CeO2), a component of catalytic
converters. They add a cerium precursor to the reaction mixture after
the BHA particles have already formed, so that it precipitates on the
surface of the BHA particles. When these particles are later heated to
800 ![]() C (the
calcination step), the dusting of precursor is converted to cerium
oxide.
C (the
calcination step), the dusting of precursor is converted to cerium
oxide.
"We were able to disperse cerium oxide very finely on the BHA
surface" so that even at 1,300 ![]() C
the combined surface area is still as high as 70 to 80 sq m per g, Ying
reported. Even more important, she added, is that the cerium oxide
crystallites grow only slightly--to 20 nm--when baked at 1,300
C
the combined surface area is still as high as 70 to 80 sq m per g, Ying
reported. Even more important, she added, is that the cerium oxide
crystallites grow only slightly--to 20 nm--when baked at 1,300 ![]() C.
"That's remarkable because unsupported cerium oxide particles tend
to grow well above 100 nm by 700
C.
"That's remarkable because unsupported cerium oxide particles tend
to grow well above 100 nm by 700 ![]() C."
C."
When the MIT researchers checked the CeO2/BHA
nanocomposite's ability to catalyze combustion of a 1% methane stream,
they observed light-off very close to their goal--at 430 ![]() C.
Moreover, the new catalyst sustained a stable, reproducible activity
beyond 1,100
C.
Moreover, the new catalyst sustained a stable, reproducible activity
beyond 1,100 ![]() C,
Ying told her audience.
C,
Ying told her audience.
Ying believes that the use of noble metals and other complex oxides might allow her group to lower the light-off temperature even further. And even if a single nanocomposite isn't found to possess all the desired attributes of a methane combustion catalyst, she envisions a possible two-stage process: up front, a catalyst like CeO2/BHA that kicks off at a low temperature; and downstream, a catalyst that can work at the highest temperatures required, like nanostructured BHA. The MIT project is being funded by a company (which Ying can't disclose) that is interested in commercializing a process for generating energy from catalytic natural gas combustion.
The method developed by Ying's group can easily be adapted to prepare many different kinds of compositions. The group now is working to develop a number of other high-temperature reactions exploiting BHA as a support.
The use of supported catalysts is not confined to the industrial
world--they can also be found in the home. For example, the bathroom
deodorizer sold in Japan since 1992 is a supported catalyst consisting
of 2- to 5-nm gold nanoparticles on iron oxide (![]() -Fe2O3),
carried on a zeolite-coated paper honeycomb. It works by oxidizing
(decomposing) odor molecules.
-Fe2O3),
carried on a zeolite-coated paper honeycomb. It works by oxidizing
(decomposing) odor molecules.
The Au/![]() -Fe2O3
nanocomposite is an outgrowth of research carried out in the 1980s by
Masatake Haruta of Osaka National Research Institute (ONRI) in Japan and
his coworkers. They showed that gold catalysts, suitably prepared and
supported on transition-metal oxides, were extraordinarily active in the
oxidation of carbon monoxide--even well below room temperature. Further
work at ONRI and elsewhere demonstrated gold's catalytic activity in the
oxidation of hydrocarbons; reduction of NOx by hydrocarbons,
CO, or H2; hydrochlorination of acetylene; and other
reactions.
-Fe2O3
nanocomposite is an outgrowth of research carried out in the 1980s by
Masatake Haruta of Osaka National Research Institute (ONRI) in Japan and
his coworkers. They showed that gold catalysts, suitably prepared and
supported on transition-metal oxides, were extraordinarily active in the
oxidation of carbon monoxide--even well below room temperature. Further
work at ONRI and elsewhere demonstrated gold's catalytic activity in the
oxidation of hydrocarbons; reduction of NOx by hydrocarbons,
CO, or H2; hydrochlorination of acetylene; and other
reactions.
Haruta, who is now a department director at ONRI, tells C&EN that a CO-selective gas sensor based on a gold catalyst has been developed and is available to interested parties. In addition, ONRI and industry are collaborating on R&D for commercializing gold catalysts that are active in the decomposition of dioxins and the direct epoxidation of propylene.
The emergence of gold as an exceptional heterogeneous catalyst came as a big surprise when Haruta and coworkers first reported their results in the late 1980s. Gold's catalytic abilities had largely escaped detection because researchers hadn't tried sufficiently small gold nanoparticles. They also hadn't dispersed the gold on the right metal-oxide supports. Once Haruta tried this combination, he discovered that the catalytic chemistry of gold changes dramatically and that the nature of the gold/metal-oxide interface is an important influence on that chemistry.
Organic/inorganic compositesThe benefits accrued by having nanoscale particles interact with a second phase also can be seen in another huge category of materials known as organic/inorganic nanocomposites. In these materials, typically, an inorganic filler is dispersed in an organic polymer matrix. The filler carries load, and its large surface area, interacting with the matrix, helps reduce the mobility of the polymer chains, according to Linda S. Schadler, an assistant professor in the materials science and engineering department at RPI. These interactions work to improve the toughness and other mechanical properties of the nanocomposite.
Nanoparticle/polymer composites are the focus of a new research
initiative at RPI involving Schadler, Siegel, and Ajayan. Schadler and
Siegel, for instance, have been studying a nanocomposite in which
titanium dioxide particles are dispersed in a commercial epoxy resin.
The resin is heated to 60 ![]() C
to make it less viscous, and the TiO2 particles (32 nm
average diameter) are added with stirring. The mixture is sonicated for
an hour to disperse the particles, after which a chemical is added to
harden the composite.
C
to make it less viscous, and the TiO2 particles (32 nm
average diameter) are added with stirring. The mixture is sonicated for
an hour to disperse the particles, after which a chemical is added to
harden the composite.
In tests conducted by Chek Beng Ng as part of his master's thesis
work, the RPI researchers compared the properties of this nanocomposite
with those of epoxy filled with an equal loading of 0.24-![]() m
TiO2 particles (a microcomposite) and pure epoxy. They found
that the nanocomposite (with 10% by weight of TiO2) could
withstand the most strain before cracking. The next strongest was the
pure epoxy, followed by the microcomposite.
m
TiO2 particles (a microcomposite) and pure epoxy. They found
that the nanocomposite (with 10% by weight of TiO2) could
withstand the most strain before cracking. The next strongest was the
pure epoxy, followed by the microcomposite.
In scratch tests, the epoxy nanocomposite was dramatically more scratch-resistant than either the pure epoxy or the microcomposite, Schadler reported in Alaska. In the nanocomposite, not only were the scratches half as deep as in the other two materials, Siegel elaborates, "but the ancillary damage was minimal. So it's a big difference." And the difference is due to the fact that the 32-nm particles have a much larger surface area and therefore can shore up a much larger volume of the polymer matrix, Siegel explains. The scratch resistance would be a big selling point for various plastic parts that need to wear well and look good, he says, not to mention automobile paints and other nanocomposite coatings.
Organic/inorganic nanocomposites also have shown promise for use in the high-temperature environment found under the hood of the automobile. In 1993, researchers at Toyota Central R&D Laboratories in Nagakutean, reported that nylon nanocomposites containing small amounts of clay (layered silicates) possessed exceptional properties. Like cutting a deck of cards, the researchers had separated the silicate stacks into individual platelets about 1 nm thick and dispersed these throughout the polymer.
The dispersed platelets dramatically increased the polymer's stiffness and strength without sacrificing its toughness. Even more important, these hybrid materials were found to tolerate higher temperatures without warping. Today, such a nylon-clay nanocomposite, fashioned into an air intake cover, is found under the hood of the Toyota Camry.
At the time of the pioneering Toyota work, Emmanuel P. Giannelis, a professor in the materials science and engineering department at Cornell University, and his coworkers were studying polymer/layered silicate nanocomposites of a different type. They were developing methods for infusing polymers into the interlayer spaces or "galleries" of layered silicates. The resulting intercalation nanocomposites have alternating polymer and silicate layers and a high degree of order. And they exhibit interesting properties that result from the confinement of the polymer chains in the two-dimensional galleries.
But because the Toyota group was able to achieve more impressive properties with the dispersed nanocomposites, Giannelis turned his attention to these materials. His group has been trying to find better ways to synthesize these nanocomposites, as well as studying how to control the interface between the organic and inorganic components.
As Giannelis tells C&EN, when silicate platelets are dispersed in the spaghetti-like mass of polymer strands, the strands that interact directly with the silicate surface adopt a different arrangement--they may stretch out, for example. This change leads to different dynamical behavior. So in essence, he says, "you have changed the properties of the polymer at the interface." And since the high surface area of the filler produces lots of interfaces, the amount of poly-mer affected by those interfaces is quite large--perhaps 60 to 70%. Thus the properties of the bulk sample are changed.
To make a successful nanocomposite, Giannelis stresses, it's very important to be able to disperse the inorganic material throughout the polymer and create those interfaces. If a uniform dispersion isn't achieved, clumps of inorganic material end up inside the polymer, which doesn't lead to desirable properties. It's also important, he says, to split the silicate "deck of cards" into individual cards because dispersing those cards allows more polymer to be affected with less filler.
But that's not as easy as it may sound because the two components aren't very compatible. Silicates are hydrophilic, Giannelis points out, and the polymers--at least the most interesting ones for engineering applications--are hydrophobic. To produce the intercalated nanocomposites, the polymer has to wet the clay particles to some extent so that it can worm its way into the galleries. But to make the delaminated or dispersed nanocomposites, a lot more wetting is required. The thermodynamics works against you in this quest, Giannelis says, so you basically have to trick nature.
One scheme to do that is described in a recent paper published by Giannelis; his collaborator Dotsevi Y. Sogah, a professor in Cornell's department of chemistry and chemical biology; and graduate students Marc W. Weimer and Hua Chen [ J. Am. Chem. Soc., 121, 1615 (1999)]. Their strategy involves chemically modifying the interlayer surfaces of the silicate to make the silicate less hydrophilic and therefore more wettable by the polymer. They accomplish this by ionically bonding an organic surfactant to the surfaces. This surfactant contains a nitroxyl function, which initiates free-radical polymerization in the presence of a suitable monomer. When the researchers heat this surfactant-modified silicate in styrene, the styrene molecules diffuse into the galleries and polystyrene chains begin growing from the bound surfactant molecules.
The Cornell team's hope was that as the polymerization proceeded and the galleries became increasingly congested with polymer chains, the silicate layers would gradually be forced apart until they were well separated, leading to a well-dispersed nanocomposite.
That's what seems to have happened. After the reaction mixture has been heated for eight hours, it solidifies completely to form a homogeneous, transparent solid. X-ray diffraction and other data suggest that silicate platelets are randomly and uniformly dispersed throughout the polystyrene matrix. Giannelis points out that a uniformly dispersed nanocomposite of this type has not previously been possible to achieve with polystyrene. "We had always formed intercalated structures, but never dispersed," he says.
This synthesis method has the advantages that it allows control of the molecular weight of the polymer and the distribution of molecular weights. And since the polymer growth process is one known as "living polymerization," it allows for additional monomers to be added to grow block copolymers. But the method is limited to polymers that can be grown by living polymerization, Giannelis notes.
One polymer that probably cannot be grown by this process is poly(methyl methacrylate) (PMMA). And since Giannelis was interested in making dispersed silicate nanocomposites using this polymer, he devised another scheme to trick nature. This one is quite a bit simpler. It involves polymerizing methyl methacrylate in an aqueous emulsion containing the powdered silicate. The water in the reaction mixture readily separates the silicate stacks into single layers.
PMMA is produced industrially using a similar emulsion process, so Giannelis' emulsion route to PMMA nanocomposites is commercially "very attractive," he believes. Another advantage of this method is that it yields PMMA nanocomposites that are transparent and colorless--two requirements for their use in paints and coatings.
When montmorillonite, a well-known silicate clay, is used as the inorganic filler for PMMA, the Cornell researchers produce a transparent nanocomposite that also absorbs UV light. This property would help to protect the polymer, which is degraded by UV light, Giannelis says. The clay filler also endows the nanocomposite with greater thermal stability.
Giannelis notes that industry has shown a lot of interest in silicate nanocomposites. But, like most product-oriented research done in industrial labs, the results rarely appear in the open literature.
At the conference in Alaska, though, Thomas P. Feist, a materials scientist at GE Corporate Research & Development in Schenectady, N.Y., gave attendees a glimpse into some of his company's research activities in the area of polymer nanocomposites. GE, Feist said, has been exploring polymer materials filled with nanosized clay particles and with carbon nanotubes.

One material of interest is poly(butylene terephthalate), a thermoplastic that is used in electrical connectors even though it is flammable. By dispersing a nanoclay in this polymer, GE scientists produced a nanocomposite with encouraging flame-retardant performance. The nanoclay improved performance in this regard better than traditional micrometer-sized fillers, Feist said.
Carbon nanotubes as fillersGE also has been interested in dispersing carbon nanotubes in polymers because certain kinds of nanotubes, by virtue of their electrical conductivity, make the nanocomposite conductive as well. Feist noted that nanotube loadings of less than 10% have been tried in a variety of engineering resins, including polycarbonate, nylon, polyesters, and poly(phenylene ether)/polyamide (PPE/PA). The nanotubes confer high conductivity at lower loadings than carbon black or micrometer-sized fillers such as carbon fibers or stainless steel fibers, he said.
In a recent Nature article [399, 210 (1999)], Paul D. Calvert, a professor of materials science and engineering at the University of Arizona, Tucson, suggests why a conductive nanotube/polymer blend might one day find its way into car bodies. "In a modern car factory," Calvert writes, "efficient spray painting of vehicles is assured by giving the paint spray an electrostatic charge, which is then attracted to the car body by the high voltage passing through it. This works fine when car bodies are made of sheet steel, but increasing use of composite materials makes it more difficult to apply an even coat."
GE has addressed this problem, Feist noted, by creating a conductive, nanotube-filled, impact-toughened PPE/PA formulation that could be used in car body panels amenable to electrostatic spray painting.
And GE is by no means the only company interested in carbon nanotubes as fillers in nanocomposites. Industrial interest in these materials is running high, and companies are collaborating with academic labs to explore their potential.
The "nanotube bug" also has bitten government agencies such as the National Aeronautics & Space Administration. Last October, NASA signed an agreement with Rice University, Houston, to work together on carbon nanotechnology. Bradley S. Files, who is head of the nanotube project at NASA's Johnson Space Center in Houston, told attendees of the Alaska conference that NASA has its eyes on Mars. "If we want to go to Mars in 10 or 15 years," Files said, we'll need to focus on new technologies, including lightweight high-strength composites, chemical sensors, conductive polymers, nanoscale devices, hydrogen storage, and other potential applications involving nanotubes.
In collaboration with chemistry and physics professor Richard E. Smalley's group at Rice, Files's group set up a laser ablation system at Johnson Space Center to produce single-walled nanotubes (SWNTs) two and a half years ago. Earlier this year, Files and coworkers also set up an electric arc system to produce nanotubes. The NASA scientists are incorporating these tubes into engineering plastics and studying their materials properties.
The widespread interest in carbon nanotubes is easy to understand. "The buzz is that the nanotube is the world's most perfect fiber, and therefore should have the best mechanical properties," says John E. Fischer, professor of materials science and engineering at the University of Pennsylvania. Researchers have performed "lots of calculations and a few experiments" addressing this question, he says, "and it appears that, indeed, they are atomically perfect and the intrinsic properties are wonderful."
Many scientists believe, as Calvert states in Nature, that carbon nanotubes "should be the ideal reinforcing fibers for composites." One reason is that these graphitic tubes have a very high aspect ratio (length-to-diameter ratio) of 1,000 or more. And although their strength hasn't been measured yet, it's expected to be two orders of magnitude greater than that of any other known material.
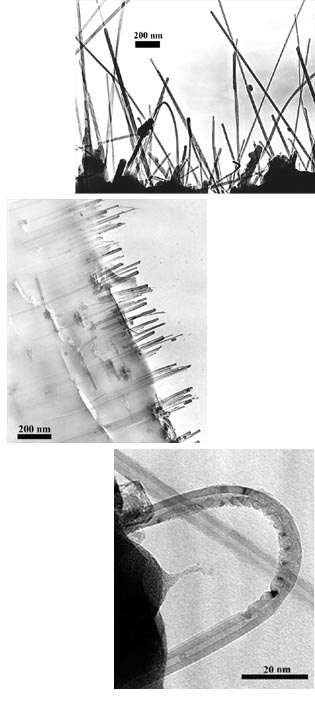
Moreover, nanotubes have been found in experiments to be much stiffer than carbon fibers, yet less brittle. Carbon fibers, which are used to strengthen materials in sports equipment, break at very low strain--less than 1% deformation, according to Otto Zhou, assistant professor of materials science in the physics and astronomy department at the University of North Carolina, Chapel Hill. Zhou and his colleagues have shown recently that nested or multiwalled nanotubes (MWNTs) embedded in a polymer matrix can withstand deformations up to 15% before they break--a big improvement over carbon fibers, Zhou says.
But "the greatest advantage of nanotubes in composites," according to a paper from the Schadler-Ajayan collaboration, "will be in the ease of processing, especially in the lack of breakdown during processing (which is a severe problem for carbon fibers). The low densi-ty of nanotubes is the other obvious advantage."
These and other considerations suggest that nanotubes have the potential to usher in a new generation of tough, lightweight, high-strength nanocomposites. But the path to fulfilling that dream is strewn with pitfalls and challenges.
To begin with, scientists haven't yet studied in detail several important issues related to the performance of nanotube composites. The first of these is nanotube dispersion and orientation. Because micrometer-length nanotubes aren't soluble in organic solvents, they usually must be mixed with polymer solutions as a suspension, which can lead to undesirable aggregations of nanotubes in the final composite. In the past year, researchers have learned how to chop long, entangled bundles of SWNTs into shorter "pipes" and functionalize these so that they are soluble in organic solvents (C&EN, Oct. 5, 1998, page 9).
But for composites, scientists are interested in using the longer nanotubes, says chemistry and physics professor Robert C. Haddon of the University of Kentucky, Lexington. If micrometer-length tubes could be solubilized, they would be easier to disperse in a matrix, Haddon believes. He tells C&EN that his group has found a way to solubilize such long tubes, although he doesn't yet understand exactly why such large objects can be dissolved. Haddon isn't ready yet to reveal details of the work.
For some applications, an alignment of the nanotubes within the matrix would be beneficial. Researchers have been exploring a number of strategies to achieve this. Last year, for example, Zhou and coworkers described a simple method for aligning nanotubes by mechanical stretching [Appl. Phys. Lett.,73, 1197 (1998)].
They first prepared a composite film containing randomly dispersed tubes by allowing a suspension of MWNTs in a chloroform solution of a thermoplastic polymer to dry by evaporation. The resulting film was then heated above the softening point and mechanically stretched by applying a constant load. The sample was allowed to cool to room temperature before the load was released. X-ray diffraction and electron microscopy studies of the sample indicated that the stretching had partially aligned about half of the nanotubes parallel to the elongation direction. However, in the case of SWNT bundles, which are more flexible and have much higher aspect ratios than multiwalled tubes, Zhou and coworkers observed no significant alignment after this procedure.
The second performance issue that concerns materials scientists in this field is interfacial adhesion. For a nanotube to serve as reinforcement, it "must be well bonded to the polymer matrix," Calvert points out. "We need the polymerized resin to stick to the tubes strongly enough so that the load is transferred to the fibers instead of the two surfaces slipping." Some studies, including an early one by Ajayan and coworkers published in 1994, indicate that the interfacial bonding is weak. In other studies, such as the aforementioned one by Zhou and coworkers, investigators have concluded that the interfacial bonding is strong. The issue remains controversial, Ajayan notes.
If adhesion is weak, scientists have raised the possibility of chemically introducing "defects" or functional groups on the nanotube's side walls to anchor them to the matrix or to other nanotubes. Defects would improve adhesion to the polymer, Ajayan says, but they could also adversely affect the nanotube's strength and electronic properties, which are of prime interest in many applications. In any case, chemists have only recently begun to take the first baby steps at functionalizing the sidewalls of nanotubes (C&EN, Jan. 11, page 31), and further progress will likely be made.
Materials scientists also are trying to understand the forces that cause nanotubes to deform in polymer composites and what these deformations can tell them about strain and load transfer. Zhou and coworkers, for instance, have stretched their composites at room temperature to the point of fracture. At the fracture surface, they observed plastically deformed tubes, which they believe indicates that there is strong interfacial bonding between the tubes and the matrix. They examined a large number of bent and buckled (crumpled) nanotubes embedded in the matrix and were able to estimate the minimum amount of strain necessary to buckle a nanotube or cause a fracture in the composite.
At Weizmann Institute of Science in Rehovot, Israel, H. Daniel Wagner's group in the department of materials and interfaces also has been studying the mechanical behavior of embedded nanotubes. From their results, they estimate that MWNT-polymer composites are at least 10 times more efficient at transferring stress than conventional fiber-based composites.
Even if nanotubes prove their mettle in composites, they face other hurdles, including cost, availability, and manufacturability. Some of these challenges were given voice at a two-day conference on "Commercialization Advances in Large-Scale Production of Carbon Nanotubes." The meeting was organized by the Boston-based Knowledge Foundation and held in April in Washington, D.C.
At the conference, Ching-Hwa Kiang, a visiting assistant professor of chemistry at the University of California, Los Angeles, enunciated a truism that must have been on the minds of many attendees: For nanotubes to be used in commercial composites, they will need to be manufactured on a large scale and at low cost.
But as the University of Kentucky's Haddon pointed out at the same meeting, "We really can't make enough of this material [nanotubes]. We can't make it in very pure form. And we don't have very good analytical techniques for understanding exactly what it is that we've made."
Haddon was referring to research quantities of nanotubes, which are available from $100 per g for impure, as-prepared material, to $1,400 per g for purified SWNTs. As Calvert points out inNature , the minimum price is still "roughly 10 times the price of gold, and so is too costly for most applications."
Most current methods of synthesizing nanotubes are not amenable to large-scale production, so a number of labs are trying to develop methods that could be scaled up. "We'd like to make a ton a day," said chemist Daniel T. Colbert of Rice University. And he thinks that goal could be achieved with a method he and his coworkers (including Smalley) are working on: the gas-phase growth of SWNTs using carbon monoxide as the feedstock.
One scientist at the Washington, D.C., conference privately confessed skepticism that nanotubes will be used in composites because they are so expensive. But many people expect the price of nanotubes to go down when large-scale production begins and demand grows. Demand is likely to pick up if nanotubes are found to offer performance advantages that other materials can't. Even if nanotubes remain pricey, their performance may warrant their use in certain niche markets, according to Zhou.
In any case, Zhou says, "It's still early in the game." After all, nanotubes were discovered only eight years ago, and research quantities became available even more recently. Zhou's research program on nanotube composites, like those at many other labs, is only about a year old. And although several speakers at the nanotube commercialization conference said they were working on nanotube composites, few had substantial results to report on these materials.
One researcher who did have substantial, though preliminary, results to report was materials physicist Apparao M. Rao of the Center for Applied Energy Research at the University of Kentucky. Rao and coworkers Frank Derbyshire, Rodney Andrews, and Terry Rantell, in collaboration with Haddon's group, have been fabricating carbon fibers reinforced with nanotubes.

Conventional carbon fibers, which are thicker than 1 ![]() m,
are themselves used as reinforcing fillers in traditional composites.
One established way to make these carbon fibers is by pyrolysis of
pitch, the carbonaceous residue from the distillation of coal tar or
petroleum.
m,
are themselves used as reinforcing fillers in traditional composites.
One established way to make these carbon fibers is by pyrolysis of
pitch, the carbonaceous residue from the distillation of coal tar or
petroleum.
To make their nanotube-filled carbon fibers, the Kentucky researchers
start with a commercially available petroleum pitch. They dissolve it in
quinoline with heating, add purified SWNTs and disperse them
ultrasonically, and later remove the solvent under vacuum. The resulting
nanotube-pitch mixture is softened by heating and extruded as 20-![]() m-thick
fibers. After oxidative stabilization, the composite fibers are
carbonized by slowly raising the temperature to 1,100
m-thick
fibers. After oxidative stabilization, the composite fibers are
carbonized by slowly raising the temperature to 1,100 ![]() C
under a flow of nitrogen.
C
under a flow of nitrogen.
Rao and coworkers then measure the tensile strength, elastic modulus (a measure of stiffness), and electrical conductivity of the nanotube-filled fibers. Compared to unfilled fibers, fibers containing 5% by weight of the nanotubes show enhancements of about 90% in tensile strength, about 150% in elastic modulus, and about 340% in conductivity, Rao told his listeners. He believes that even more impressive properties will be measured as improved nanotube/carbon fiber composites are prepared.
Researchers also are using nanotube fillers to increase the electrical conductivity of polymers that emit light when electrically stimulated. Werner J. Blau, a professor of physics at Trinity College Dublin in Ireland, and his coworkers added MWNTs to the luminescent polymer poly(m-phenylenevinylene-co-2,5-dioctoxy-p-phenylenevinylene) (PmPV). At the Alaska conference, he reported that when 10% by weight of nanotubes is dispersed in this polymer, the conductivity increases by up to 10 orders of magnitude--an even greater enhancement than his group reported in their preliminary paper on the work [Adv. Mater., 10, 1091 (1998)].
In addition, Blau noted that the nanotubes increase the luminescence. "The optical properties of the nanocomposite are quite remarkably different from what you expect them to be," he said.

His group, which is collaborating with Ajayan's at RPI and physicist David L. Carroll's group at Clemson University, Clemson, S.C., has made attempts to fabricate light-emitting diodes incorporating the nanotube composite. But so far, he said in Alaska, the devices glow for only a few minutes. He also revealed that the nanocomposite exhibits some nonlinear optical properties, but more work is needed to nail down this aspect.
In the course of preparing the nanocomposite, Blau and coworkers found another use for PmPV. He pointed out that the backbone of this soluble polymer tends to coil, forming a helical structure. The helix has a diameter of 1.4 to 2 nm, which, he noted, "fits nicely around a single-walled nanotube." Blau thinks the polymer may wrap itself around the nanotube and hold it in suspension in the polymer solution. This is a great way to clean up as-prepared nanotube samples, which usually contain a large fraction of soot and other impurities. When the impure nanotube sample is added to the PmPV solution and sonicated a few minutes, he said, the nanotubes dissolve, leaving behind the amorphous crud.
The Dublin team is not the only one that has discovered the benefits of polymer-wrapped nanotubes. At Hong Kong University of Science & Technology, assistant professor of chemistry Ben Z. Tang and postdoc Hongyao Xu have been polymerizing phenylacetylene in the presence of short nanotubes. The soluble product--poly(phenylacetylene) (PPA)--wraps around the tubules, apparently keeping them solvated in solution [Macromolecules, 32, 2569 (1999)].
Tang and Xu have observed that such a nanotube/PPA solution responds to laser pulses "in a strikingly different way" than does a solution of pure PPA. As the laser pulses become more intense, the nanotube/PPA solution becomes more opaque, cutting off light transmission. Nothing like this happens with ordinary PPA solutions. "Clearly," the researchers write, "the nanotubes have endowed the [nanotube/PPA] with optical limiting power."
They believe such nanocomposites may find "an array of potential applications in optics-related, especially laser-based, technologies." Currently, Tang tells C&EN, "we are trying to use our nanocomposites to build photovoltaic devices that directly convert light to electricity."
The dreams abound, but will nanotubes actually make a dent in future nanocomposites? Obviously, it's too early to know, but RPI's Siegel certainly thinks so. "I think there's a tremendous opportunity because carbon nanotubes have very interesting properties," he says. "But we're going to need to understand much, much better the interaction of the tubes with the matrix and how to modify those interactions."
Siegel believes the "exciting potential" of nanotubes will
drive them toward commercial viability. The scale-up issue, though, is a
crucial one: "Nothing impacts society unless it's scaled up,"
he says. "You have to be able to scale up and you have to be able
to do it economically." But since nanotubes have so much
technological promise, he adds, "it's a little hard to believe that
some of it is not going to come to fruition."![]()
Chemical & Engineering News
Copyright © 1999 American Chemical Society