Research: Corrosion Inhibition of Thin Metal Films using Surfactants
Caroline Murira and Hannes Schniepp
Advisors: Ilhan A. Aksay
In industrialized countries, material degradation due to corrosion costs 2-3% of the gross national product per year [1]. These costs can be reduced significantly by engineering effective corrosion inhibitors for metal surfaces. Surfactants are one example of molecules that can be used as corrosion inhibitors. Due to their amphiphilic nature they adsorb on surfaces and form aggregates with different morphologies (see Figure 1) [2,3,4], which potentially provide different amounts of corrosion inhibition. The goal of this work is to investigate how different surfactant aggregates on metal surfaces affect corrosion inhibition and use this information to make better corrosion inhibitors.
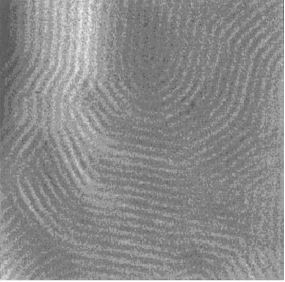
Figure 1: 200 nm x 200 nm AFM image of surfactants on annealed gold substrate [4]
Currently we are studying the corrosion inhibition of iron using sodium dodecyl sulfate (SDS) and the corrosion inhibition of copper using cetyltrimetylammonium bromide (CTAB). SDS and CTAB have been shown elsewhere to inhibit corrosion of iron (steel) and copper respectively on a macroscopic scale using the weight loss method [5] or electrochemical methods [6]. To get a better understanding of the mechanism of corrosion inhibition and specifically how surfactant aggregates influence inhibition, we are studying corrosion on a microscopic and nanoscopic scale using an optical microscope and an atomic force microscope (AFM).
The corrosion inhibition rate of the surfactants is studied microscopically in the well-defined corrosion cell shown in Figure 2. Metal thin films are prepared on a silicon wafer and they are exposed to an acidic solution containing different concentrations of surfactant. The inhibition rate is determined by the time it takes for the metal (iron or copper) to dissolve. The morphology of surfactant aggregates formed on the metal surface at concentrations corresponding to those used for the corrosion inhibition experiments is then investigated using an atomic force microscope (AFM).
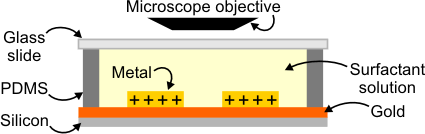
Figure 2: Optical microscope experimental setup.
Current results on the copper studies show an increase in dissolution time of copper thin films as solution concentration is increased, until approximately the bulk CMC of CTAB (0.9 mmol/lit) is reached. The morphology of surfactant aggregates on the surface is expected to change as the concentration is increased and by AFM imaging, these changes in structure are being determined.
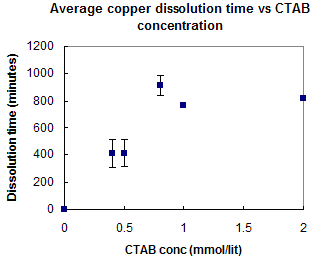
Figure 3: Graph showing inhibition rate of CTAB on copper at different surfactant concentrations.
AFM imaging of SDS on iron films was studied by Anderson Shum [7] and the images he obtained indicate the presence of surfactant aggregates on the iron as shown in Figure 4. Corrosion inhibition experiments of SDS on iron are ongoing.
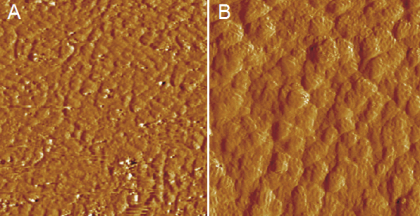
Figure 4. 500 nm x 500 nm AFM image of iron in: A.) water B) 10 mmol/lit SDS. There is no signature of surfactants in A.) which are visible in B.) as the round features on the iron grains.
References
1. S. Lyon, Nature 427 406 (2004).
2. S. Manne, J.P. Cleveland, H.E. Gaub, G.D. Stucky, P.K Hansma, Langmuir 10 4409 (1994).
3. S. Manne, H.E Gaub, Science 270 1480 (1995).
4. M. Jaschke, H.J. Butt, H.E. Gaub, S. Manne, Langmuir 13 1381 (1997).
5. M.N. Shalaby, M.M Osman, A.A El Feky, Anti-Corrosion Methods and Materials 46 254 (1999).
6. H. Ma, S Chao, S. Zhao, X Liu, D Li, J. Electrochem. Soc. 184 B482 (2001).
7. A. Shum, Senior Thesis Princeton University (2005).
For more information, please contact C. Murira. See the Ceramic Materials Laboratory web page for more information about our group and its research.
![]()
![]()
![]() © 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.