Research: Guiding the Self-assembly of Hierarchically Structured Materials
K. Jared Tatum and Jaehun Chun
Advisors: Dudley A. Saville and Ilhan A. Aksay
Collaborators: Jun Liu, Sandia National Labs (now at Pacific Northwest National Laboratory)
Since self-assembled materials mesoscopic materials were first patented by researchers at Sylvania Electric Products, Inc. in 1971 [1] and re-introduced by Mobil scientists in 1992 [2], considerable effort has been made to develop techniques to control the orientation of the nanometer- and micrometer-level morphologies. The work in our lab focuses on self-assembled mesoporous ceramics, the synthsis of which entails mixing the desired inorganic alkoxide with a solution of structure directing agent (i.e., surfactant or block co-polymer) and the appropriate catalyst for gelation of the inorganic. Specifically, our goal is to control the structure of continuous mesophases over large length-scales which would increase nanochannel accessibility and make the materials more amicable for separation processes, catalytic supports, and nanofluidic devices.
To orient mesoscopic silica on both the nanometer and micrometer length scales, we have employed anisotropic substrates [3], confinement [4], shear fields, and AC and DC electric fields [4,5] to control the orientation of mesoscopic silica thin films at both the solid-liquid and air-water interfaces. While each of the techniques have been shown to promote a preferred orientation, electric fields are the most promising since they are capable of inducing mesophase orientation in widely tunable geometries.

Figure 1: Mesoscopic silica is a model example of a hierarchically structured material
We use mesoscopic silica, synthesized by mixing an acidified cationic surfactant solution with a silicon alkoxide, as a model mesophase system (Fig. 1). [6] The self-assembly process is divided into three distinct regimes: 1. The nucleation period characterized by the presence of spherical micelles; 2. The aggregation period characterized by the growth of particles and thin films via micelle accretion; and 3. The induction period characterized by a disorder-order transition from spherical micelles to a hexagonally packed mesophase. [5,6]
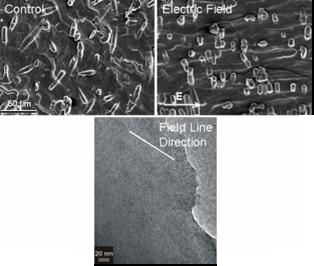
Figure 2: Application of electric fields parallel to mesoscopic silica air-water thin films results in nanometer and micrometer morphologies showing a preferred orientation parallel to the field lines.
Mesoscopic silica thin films grown at the air-water interface under the influence of electric fields show a preferred orientation parallel to the field lines across multiple length scales. The ability to achieve this orientation over large areas and within a continuous phase can greatly enhance the usability of the channels once the organic template is removed via calcination.
For more information on this research topic, please contact K. Jared Tatum. Additional information concerning the CML can be found on our website.
References
1. V. Chiola, J.E. Ritsko, C.D. Vanerpool, “Process for Producing Low-Bulk Density Silica" U.S. Patent #3,556,725 (1971)
2. C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359 710 (1992)
3. I.A. Aksay, M. Trau, S. Manne, I. Honma, N. Yao, L. Zhou, P. Fenter, P.M. Eisenberger, S.M. Gruner, Science 273 892 (1996)
4. M. Trau, N. Yao, E. Kim, Y. Xia, G.M. Whitesides, I.A. Aksay, Nature 390 674 (1997)
5. A.Y. Ku, “Guided Self-Assembly and Directed Restructuring of Mesoscopic Silica using Electric Fields” Ph.D. Thesis, Princeton University (2004)
6. N. Yao, A.Y. Ku, N. Nakagawa, T. Lee, D.A. Saville, I.A. Aksay, Chem. Mater. 12 1536 (2000)
![]()
![]()
![]() © 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.