Research: Orientational Order of Molecular Assemblies on Inorganic Surfaces
Jaehun Chun
Advisors: Dudley A. Saville and Ilhan A. Aksay
Collaborators: Je-Luen Li and Roberto Car, Department of Chemistry, Princeton University
Orientational orders of nano-structures have been observed in molecular self-assemblies such as surfactant micelles and protein fibrils. The self-assembled surfactant molecules such as cationic cetyltrimethyl ammonium chloride (CTAC) [1,2], non-ionic polyethylene oxide alkyl ethers (C12E9) [3], and de novo protein fibrils display a unique directionality on HOPG (Highly Oriented Pyrolytic Graphite) surfaces [4], as shown in the Atomic Force Microscope (AFM) images below. Our goal is to understand the underlying physics leading to these orientational orderings, which is essential for controlling the structures on nanometer length scales.
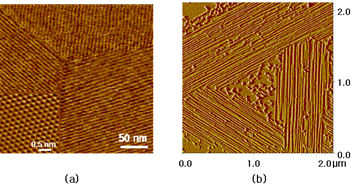
Figure 1: AFM images of (a) cationic CTAC micelles [1] and (b) protein fibrils [4] on HOPG surface. The distinct orientational orderings of molecular assemblies are shown in both images. Note that there are misaligned protein fibrils which have relatively shorter lengths in (b)
We postulate that the orientational ordering shown in Fig.1 is driven by an “anisotropic van der Waals interaction” between the molecular assembly and anisotropic inorganic surface. Although an energy difference for different orientations at molecular level exists, it is too small to account for the orientational order of the molecular assemblies.[5] Thus, our approach is appropriate to capture an essential feature on the orientational ordering of molecular assembly, in spite of the limitation to address the observed dynamic behavior of the molecular assembly at short time scale.[5]
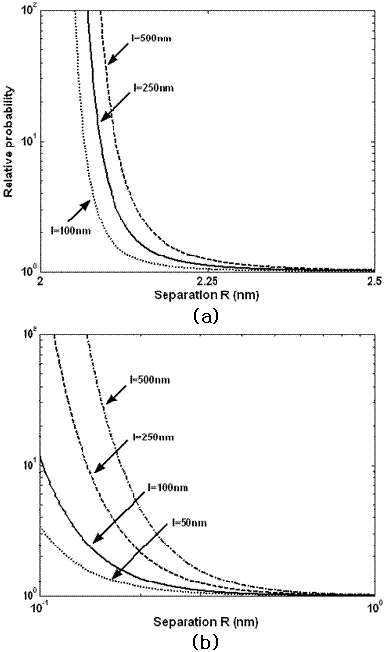
Figure 2: The relative probability or preference for the orientational ordering on the (a) micelle and (b) protein fibril. The preference is very large which suggests the strong tendency for the alignment. The preference depends on the separation between the assembly and surface as well as the length of the assembly.
The anisotropic van der Waals interaction stems from the dielectric anisotropy of the (ordered graphite) surface. The anisotropic nature gives rise to a van der Waals torque, which leads to the orientational ordering. Thus the orientational ordering of the molecular assembly can be described by an orientational probability. Detailed mathematical analysis based on the Lifshitz methodology enables us to calculate the anisotropic van der Waals interaction and resulting torque. It is shown that the orientational ordering is proportional to the length of the assembly. The analysis (Fig. 2) suggests that the micelle and the protein fibril disclose strong tendencies for alignment, i.e. at least 10-100 times larger preference to one direction, which are in accord with the experimental observations. More importantly, our study shows the existence of a critical assembly size for the orientational ordering, which is clearly observed in the experiment (Fig. 1(b)).
For more information on this research topic, please contact Jaehun Chun. Additional information concerning the CML can be found on our website.
References
1. I.A. Aksay, M. Trau, S. Manne, I. Honma, N. Yao, L. Zhou, P. Fenter, P.M. Eisenberger, S.M. Grunner, Science 273 892 (1996)
2. S. Manne, H.E. Gaub, Science 270 1480 (1995)
3. H.N. Patrick, G.G. Warr, S. Manne, I.A. Aksay, Langmuir 13 4349 (1997)
4. C.L. Brown, I.A. Aksay, D.A. Saville, M.H. Hecht, J. Am. Chem. Soc. 124 6846 (2002).
5. D.A. Saville, J. Chun, Je-Luen Li, Hannes C. Schniepp, Roberto Car, I.A. Aksay, Phys. Rev. Lett. submitted (2005).
![]()
![]()
![]() © 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.