Research: Neutral pH Catalysis of Silica Condensation
Douglas H. Adamson1 and Daniel M. Dabbs
1Princeton Institute for the Science and Technology of Materials
In collaboration with Daniel E. Morse, UCSB
We have successfully synthesized a catalytically active polymer inspired by the protein Silicatein. This functionalized block co-polymer catalyzes the formation of solid poly(silicate) from tetraethoxysilane (TEOS) under neutral pH and ambient temperatures.[1] Polymer synthesis was guided by the composition of the natural protein and its proposed mechanism.[2-5]
Patterned ceramic structures can be synthesized using organic molecules—surfactants and block copolymers—as templates for the nucleation and growth of the inorganic phase.[6] There is an extensive literature on the silicification of these templates using TEOS as the source of silicon oxide. In the absence of catalyst the hydrolysis and condensation of TEOS are slow processes. Catalyzing the formation of silica from the TEOS precursor can involve high temperature, extreme pH conditions, or both, conditions which may be deleterious to included materials or to organic templates needed to build patterned silica structures. Synthetic templates that are stable under low pH exist,[6] but are limited when compared to natural templates that encourage the nucleation and growth of inorganic oxides at near neutral pH and temperatures at or below typical room temperature.[2-5]
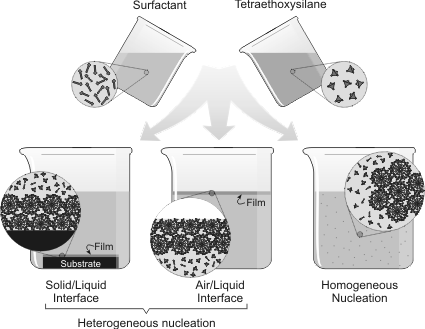
Generalized mechanisms for forming structured silica through the interaction of a structure-directing agent (surfactant) and silicon alkoxide (TEOS). Incorporating a catalytic agent within the template structure speeds the silicification of the template.[6]
The protein Silicatein has been reported[2-4] to catalyze the production of poly(silicate) from TEOS at neutral pH and under ambient temperature. The protein was isolated from the marine sponge Tethya aurantia, a sponge whose structure is defined by silica needles that constitute up to 75% of its dry weight. Occluded in these needles are protein filaments that direct and catalyze their synthesis. About 70% of this organic component is the protein Silicatein. Our goal is to emulate the natural system by building a non-peptide-based synthetic analog that can be incorporated within a structure-directing agent used to produce structured ceramic materials.
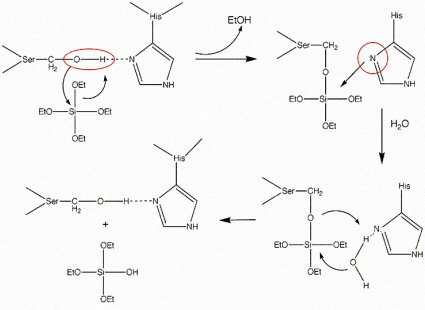
Mechanism for the catalysis of TEOS hydrolysis by the silicatein molecule.[4]
By employing site-directed mutagenesis the identity of the amino acids critical to the condensation of TEOS was determined[2]. The hydroxyl group of serine-26 and the imidazole of histidine-165 were shown to play key roles in the condensation of TEOS.[4] These functionalities were proposed to participate in the formation of a transitory pentavalent silicon species stabilized through a donor bond to the imidazole nitrogen. This proposed mechanism for the condensation of TEOS was analogous to the mechanism of peptide bond hydroylsis by the homologous protease cathepsin L. Thus, we have incorporated both hydroxyl functionality and a nitrogen containing heterocycle in our mimic and have demonstrated that it does catalyze the condensation of silica by mimicking the functionality of a natural protein. To our knowledge this is the first example of silica produced using a bio-inspired, non-peptide catalytic system.
References
1. D.H. Adamson, D.M. Dabbs, D.E. Morse, I.A. Aksay unpublished research (2004).
2. K. Shimizu, J.N. Cha, G.D. Stucky, D.E. Morse, Proc. Nat. Acad. Sci. 95 6234 (1998).
3. J.N. Cha, K. Shimizu, Y. Zhou, S.C. Christiansen, B.F. Chmelka, G.D. Stucky, D.E. Morse, Proc. Nat. Acad. Sci. USA 96 361 (1999).
4. Y. Zhou, K. Shimizu, J.N. Cha, G.D. Stucky, D.E. Morse, Angew. Chem. Int. Ed. 38 [6] 779 (1999).
5. J.N. Cha, G.D. Stucky, D.E. Morse, T.J. Deming, Nature 403 289 (2000).
6. D.M. Dabbs, I.A. Aksay, Annu. Rev. Phys. Chem. 51, 601-22 (2000).
For more information, please contact Daniel Dabbs. See the Ceramic Materials Laboratory web page for more information about our group and its research.
![]()
![]()
![]() © 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.