Research: Adsorption of Surfactant Aggregates at the Liquid/Solid Interface
Hannes C. Schniepp, Ho-Chung Shum
Advisors: Ilhan A. Aksay and Dudley A. Saville
The process of surfactant adsorption at the liquid/solid interface is crucial for many applications, such as detergency, lubrication, corrosion inhibition and colloid stabilization. Furthermore, micellar surfactant aggregates at interfaces are interesting model systems of self-assembled and self-healing nanostructures. Depending on several parameters such as the used surfactant type, the ionic strength, the pH and the surface material, these surface aggregates, called hemimicelles, have different geometries, such as spheres, hemi-spheres, cylinders, hemi-cylinders or flat layers. We study this adsorption process by atomic force microscopy (AFM) to get new insights in its dynamics as well as the underlying interactions. Traditionally, most experiments of this kind have been performed on either mica or graphite. We aim to work on materials that are more interesting for applications, for instance metals.
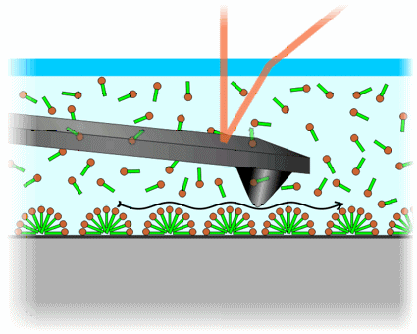
Figure 1: Schematic of liquid-cell AFM using electrostatic double-layer repulsive forces (not to scale). The AFM tip does not touch the surface of the substrate (grey) but hovers above the surface aggregates (in this case hemi-cylindrical surfactant micelles) without destroying them.
AFM is currently the only technique that allows visualizing the morphology of these structures on a variety of materials while they are in liquid environment [1]. Guided by the repulsive forces of the electric double layer close to the interface, the AFM probe hovers above these very soft and fragile aggregates without destroying them (see figure 1). The achieved resolution is high enough to image the smallest micellar aggregates with a lateral extent of about 5 nm. The acquisition time of one image is on the order of one minute, which also allows for dynamical studies.

Figure 2: 400 nm AFM scans of Cetyltrimethylammonium chloride (CTAC) hemi-cylinders on a graphite surface. The images are taken at intervals of 70 sec. and show a dynamic competition between two patches of different micelle orientation.
Figure 2 shows an animated series of four images that were acquired on a graphite surface that is immersed in a 10 mM solution of Cetyltrimethyl-ammonium chloride (CTAC) surfactant. The images are all taken from the very same area of the sample, in intervals of 70 seconds. The parallel lines with a spacing of about 5 nm represent hemi-cylindrical micellar aggregates of surfactant on graphite. All images show two areas with different orientations of the lines, making an angle of 120°. The image series documents a lively competition between the two areas, illustrating the dynamic nature of these aggregates. Based on this experimental evidence, an advanced model of the adsorption process was developed that takes into account interactions on the atomic scale as well as on the mesoscopic scale [2].
References
1. I. A. Aksay, M. Trau, S. Manne, I. Honma, N. Yao, L. Zhou, P. Fenter, P. M. Eisenberger, and S. M. Gruner, Science 273, 892 (1996).
2. D. A. Saville, J. Chun, J.-L. Li, H. C. Schniepp, R. Car, and I. A. Aksay, submitted to Phys. Rev. Lett. (2005).
For more information, please contact H. Schniepp. See the Ceramic Materials Laboratory web page for more information about our group and its research.
![]()
![]()
![]() © 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2006 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.