Research: Surfactant Templated Mesoscopic Ceramics
Shilpa Bhansali and Daniel M. Dabbs
The development of the surfactant-templated silicate MCM-41 in 1992 [1] sparked increased research into the synthesis and characterization of mesoporous inorganic solids.[2] This is in part due to the plethora of potential applications for mesoporous materials across a wide variety of uses, including areas such as filtration, energy and data storage, optoelectronics, and catalyst supports.
In our laboratory, the L3 (sponge) phase formed by the quasi-ternary system of cetylpyridinium chloride, CpCl/ hexanol/ HCl (aq) has been successfully utilized as a template for the cooperative self-assembly of silica.[3] The L3 liquid crystal is composed of an organized bilayer arranged in a random, isotropic configuration such that the bilayer divides the solvent into two equivalent volumes.[4]
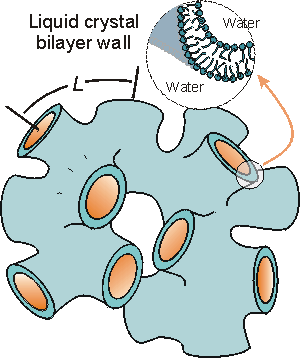
Schematic of the L3 phase (sponge) structure. The characteristic dimension L increases with increasing solvent content, resulting in a 'tunable' liquid crystalline structure [4].
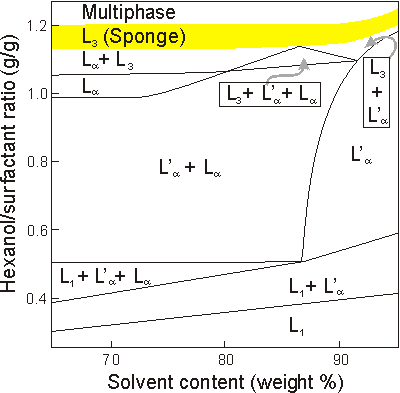
Schematic phase diagram of the quasiternary system cetylpyridinium chloride/hexanol/1 wt % NaCl solution: L1, micellar; L, "classic" lamellar phase; L', Helfrich lamellar phase; L3, sponge phase. The vertical axis depicts the connection between the cosurfactants hexanol and CpCl, and the horizontal axis reflects changes in solvent composition [8].
The key feature of our silicified L3 system is the ability to tune the mesopores (6-35 nm diameter) by changing the solvent fraction (the scaling law) in contrast to the more rigid MCM-type materials. In addition, the L3 phase solution has a viscosity comparable to that of water, facilitating the addition of inorganic precursors, the addition of which is normally a problem in the templating of the more viscous hexagonal and lamellar phases.[5]
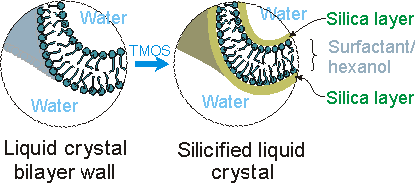
Tetramethoxysilane (TMOS) is added to the aqueous liquid crystal phase, and preferentially condenses to form silicate layers on the amphiphilic headgroups. Silicification of the L3 liquid crystalline phase maintains the connected channel structure, resulting in an optically transparent mesoporous structure.
Subsequent solvent-based supercritical drying of the silicified monoliths has successfully preserved the structural integrity yielding low density, optically transparent aerogels with high surface area and randomly oriented interconnected mesopores.
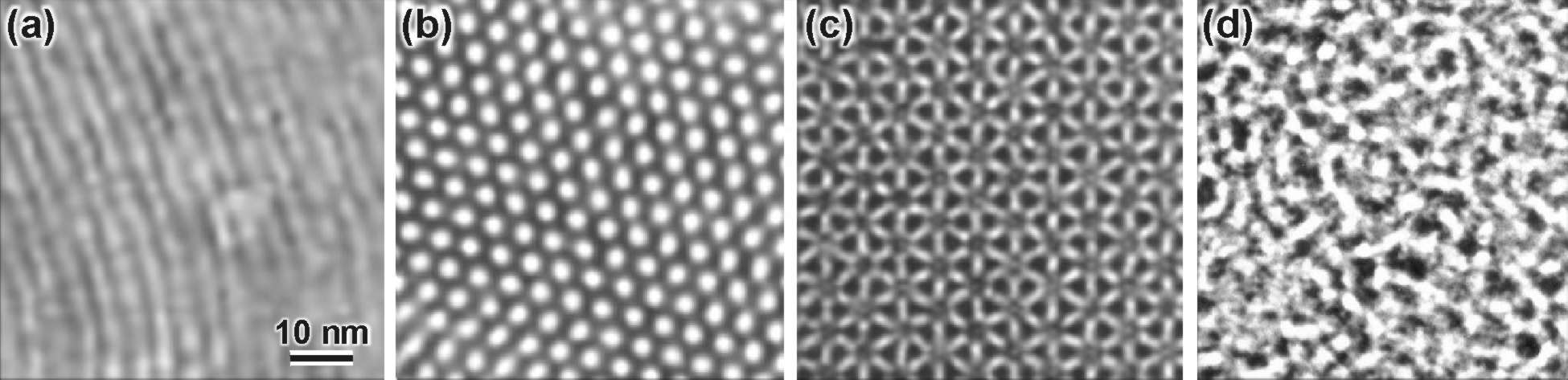
TEM images of silicified liquid crystals: (a) lamellar phase; (b) hexagonal (MCM41); (c) cubic phase; (d) L3 phase. Silicification of the L3 phase preserves the isotropic and continuous channel network of the liquid crystal (Images a-c from [9]; image d from [3].)
Unlike conventional aerogels, our material can be reinfiltrated with liquid media while remaining structurally intact. This robustness is attributed to the narrow pore size distribution of the silicified L3 system. As this material is optically transparent, the pores of our monoliths have been reinfiltrated with photocurable monomers to form a composite material for photonics applications such as optical storage.[6]
Thin films of silicified L3 have been successfully cast on glass substrates. Atomic Force Microscope (AFM) imaging has revealed the overall structure of the L3 film. The channel sizes of the L3 film for different solvent fractions have been demonstrated to follow the scaling law.[7]
We are interested in using the sponge phase for templating other metal oxides. It is envisaged that the intrinsic interconnectivity of the mesopores in these materials combined with functional properties of the oxides, will be invaluable for a wide variety of uses.
References
1. J.S. Beck et al., J. Am. Chem. Soc. 359 (1992).
2. D.M. Dabbs and I.A. Aksay, Ann. Rev. Phys. Chem. 51 601-22 (2000).
3. K.M. McGrath, D.M. Dabbs, N. Yao, I.A. Aksay, S.M. Gruner, Science 277 (1997).
4. G. Porte, J. Marignan, R. Bassereau, J. May, J. Phys France 49 (1988).
5. K.M. McGrath, D.M. Dabbs, N. Yao, K.J. Edler, I.A. Aksay, S.M. Gruner, Langmuir 16 [2] 398-406 (2000).
6. A.-S. Malik, D.M. Dabbs, I.A. Aksay unpublished research.
7. J.M. Jarvis, I.A. Aksay, J.D. Carbeck, S. Bhansali, D.M. Dabbs, unpublished research.
8. K.M. McGrath Langmuir 13 [7] 1987-95 (1997).
9. M.D. McGehee, S.M. Gruner, N. Yao, C.M. Chun, A. Navrotsky, I.A. Aksay, Proc. 52nd Ann. Mtg. MSA, eds.: G. W. Bailey and A. J. Garratt-Reed, (Microscopy Society of America, 1994) pp. 448-49.
For more information, please contact Daniel M. Dabbs.
![]()
![]()
![]() © 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.
© 2004 Princeton University, Ceramic Materials Laboratory.All Rights Reserved.