| |
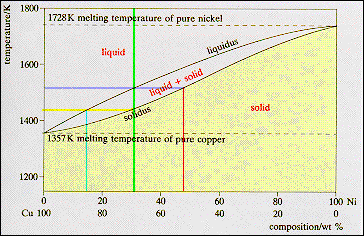
For any
other composition the alloy has a range of melting temperatures. The alloy
indicated by the vertical green line has the composition Cu - 30 wt% Ni. This
alloy starts to solidify at 1513 K, the temperature where the composition
line crosses the liquidus line. The first solid has the composition: Cu - 48
wt % Ni (red line) the point at which the isotherm crosses the solidus line.
The alloy then enters a range of temperatures where solid and liquid are in
equilibrium but there compositions change, the liquid moving down the liquidus
line and the solid down the solidus line. As the temperature is reduced the
quantity of liquid decreases and the quantity of solid increases. Solidification
is complete at the temperature where the composition line crosses the
solidus line. The final liquid has the composition Cu - 14 wt % Ni. The final
solid has the composition Cu - 30 wt % Ni as atoms must be conserved. |
|