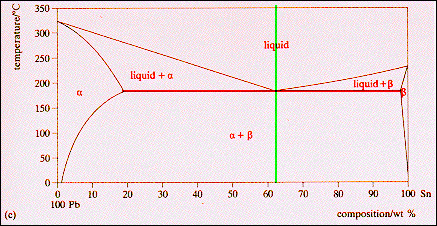
| |
Some
materials have a limited solid state solubility, the example shown below being
the Lead-Tin binary alloy system. Above 325 C these two materials will mix
as liquid over the full range of compositions. Below the Eutectic isotherm
(red) at 183 C the elements have a limited solid solubility and when this limit
is passed two phases, a + b, are both present in equilibrium. The vertical
green line identifies the Eutectic alloy composition, Pb-61.9 wt % Sn. This
alloy has a unique melting point (as do the two elements Pb {327 C} and Sn{232
C}). At the lead rich end of this binary alloy a large composition zone
is associated with the a-phase. The
maximum solid solubility of tin in lead occurs at the eutectic temperature (183
C) and the a-phase at this temperature
has the composition Pb-19.2 wt % Sn. Similarly, at the tin rich end of the
composition range, lead has its maximum solid solubility in tin at the eutectic
temperature and the b-phase at this
temperature has the composition Pb-97.5 wt % Sn (or Sn-2.5 wt % Pb). In
the single phase regions the solid alloy is more ductile than in the two phase
region as the interphase boundaries make plastic deformation more difficult. |
|