| |
|
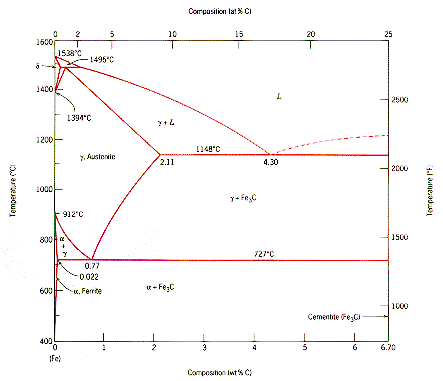
The interstitial
alloy between carbon and iron has a complex phase diagram. A compound,
Fe3C with the fixed composition Fe-6.7 wt % C bounds one end
of this diagram and pure iron the other end. Pure iron can have two different
crystal structures as its temperature is increased from room temperature to
its melting point. At room temperature it is body-centered cubic, between 912
and 1394 C if is face- centered cubic, and between 1394 and its melting point
at 1538 C it returns to body-centered cubic. |
|
| |
Low carbon
steel alloys have a composition close to the Eutectoid composition: Fe
- 0.77 wt% C. The transition that determines the micro-structure of the alloy
is a solid-state process: Austenite <-> Ferrite + Cementite
Austenite is stable above
727 C and is an interstitial solid solution of carbon in the face-centered
cubic iron lattice, Ferrite is a stable interstitial solid solution of carbon
in body-centered cubic iron below 727 C, and cementite is the ceramic-like compound
Fe3C. In going through the eutectoid reaction temperature,
carbon is displaced from the metal lattice to form the cementite compound. |
|
|
|
|
|
|
|
|
|
|