| |
Light
alloys are interesting for human-powered machines due to their low density.
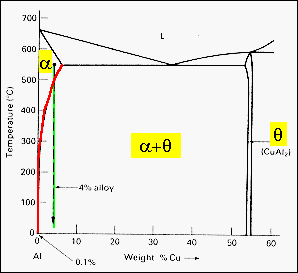
The phase diagram shown is a partial diagram for the Aluminum-Copper system
showing the aluminum rich end. The composition of a typical structural alloy
is shown by the vertical green bar on this eutectic-type diagram. If this alloy
is cooled from the liquid state to about 550 C, it has formed a polycrystalline
solid that is a substitutional solid solution of copper in the aluminum
face centered cubic matrix. The red solvus line is the limit of copper solubility
in the aluminum at different temperatures.
As the alloy is
cooled below about 500 C, the solvus line is crossed and copper starts to precipitate
as the q-phase, the alloy then
has a two phase structure with the a-solid
solution of Cu in Al forming the matrix in which the intermetallic CuAl2
compound is precipitated as small particles. The presence of this
second phase increases the yield strength of the alloy and reduces its ductility.
This alloy was developed for the structure of airships and continues
to be a useful structural material. Modification of the alloy by other alloying
additions is also common. Heat treatment can also be used to modify the
mechanical properties. |
|
|
|
|
|
|
|
|
|
|
|