| |
Iron
carbon alloys are also sensitive to the temperature at which they are formed,
the rate at which they are cooled to the transformation temperature, and for
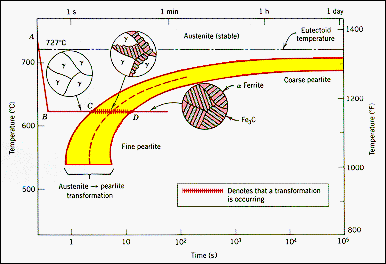
non-equilibrium states subsequent thermal treatment. The diagram and photographs
show the microstructural effects of an isothermal transformation from austenite
to pearlite. The process is represented on a Time-Temperature-Transition
curve and shows that the g - a transformation process does not start immediately.
Once started the transformation takes some time to go to the new
equilibrium state, pearlite. Once the transformation is complete the pearlite
is stable. |
|
|
|
|
|
|
|
|
|
|
| |
|
From:
Callister, Wiley (1994), & Schafer et al, "The Science and Design of
Engineering Materials," McGraw Hill (1999) |
|
| |
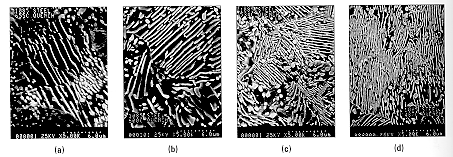
The microstructure
of the pearlite depends upon the transformation temperatures which
are: (a) 665 C, (b) 600 C, (c) 534 C, and (d) 487 C. The morphology is
lamella for each case but the inter-lamella spacing and width are smaller at
lower temperatures. |
|
|
|
|