|
|
|
|||||||||||||||
Phase Diagrams |
|||||||||||||||||
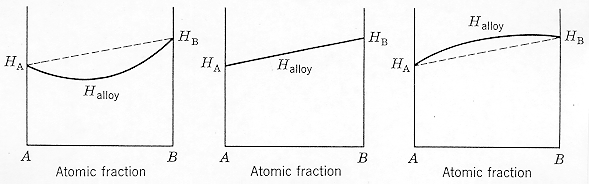
· Three cases exist for the enthalpy
curves depending on bonding strength. |
|||||||||||||||||
 |
|||||||||||||||||
From:
Brophy, et al., |
|||||||||||||||||
· If A-atoms bond more strongly
with B-atoms than with A-atoms, the enthalpy of the mixture is reduced (negative
deviation). |
|||||||||||||||||