 |
 |
 |
 |
 |
 |
 |
 |
 |
|
|
|
|
|
|
|
|
|
|
|
|
Al2O3,
Aluminum Oxide, Corundum, Sapphire |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
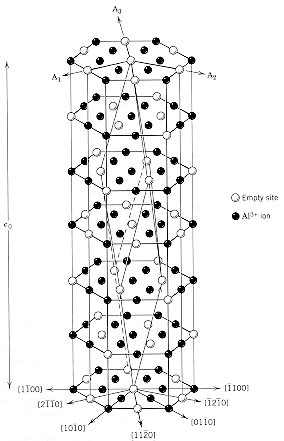
Aluminum
oxide is a ceramic compoundwith a hexagonal crystal lattice. The oxygen
anions define a hexagonal close packed structure and the aluminum cations
occupy 2/3 of the octahedral sites in the hcp lattice. Because of the high
charge (3+) on the cations, the aluminum ions want to have the maximum
spacing possible in the structure. The structural unit cell is shown in
the diagram. Because of the empty Al-sites in the basal planes of the cell,
the structure repeats itself every six layers, giving a c0
value of 1.299nm. |
|
|
|
|
|
|
|
|
|
|
|
From:
Chiang, Birnie III, and Kingery, "Physical Ceramics," Wiley, MIT (1997) |
