|
|||||||||||||||
|
|||||||||||||||
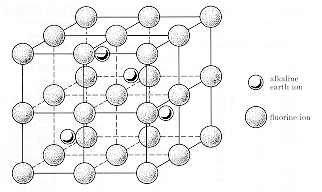
| Calcium
fluoride is an ionic crystal with the fluorine anions in a simple cubic
array and calcium cations in half of the cubic sites of the structure.
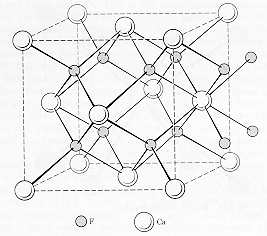
The calcium ions can also be thought of as being on an "expanded" fcc lattice
with the fluorine ions causing their lattice separation to be 0.39 nm.
This representation is shown in the lower diagram. The unit cell has 4
Ca2+ ions and 8 F1-
ions.
The material is transparent in the visible spectral region, and shows electronic optical adsorption in the ultra violet and lattice optical absorption in the infrared. |
|||||||||||||||
|
|
|||||||||||||||
| From: Brown, "The Physics of Solids," Benjamin (1967) | |||||||||||||||
 |
|||||||||||||||
| From: Kingery, Bowen and Uhlmann, "Introduction to Ceramics," Wiley (1976) | |||||||||||||||