|
||||||||||
|
||||||||||
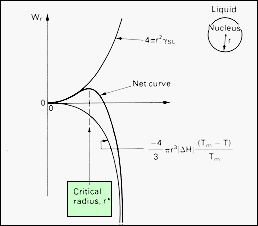
| The
formation of a second phase involves the interplay of two contributions
to the Gibbs function of the system. As the new phase forms, its radius,
r, increases, and its surface increases as r2.
The surface energy of the nucleus increases as 4pr2g,
where g
is the interphase surface energy. The new phase stable below the transformation
temperature, so that the volume energy of the nucleus is less than that
of the same volume of the initial phase. The volume energy term therefore
decreases the Gibbs function of the system as r3.
For the nucleus of radius r, the volume energy is - (4/3)pr3(DGV),
where DGV
is the volume energy decrease upon creating the new phase.
As
shown, initially the surface energy dominates the process of nucleation
and a nucleus of radius
|
||||||||||
 |
||||||||||
| From:
Ashby and Jones,
"Engineering Materials 2," Pergamon (1986) |
||||||||||