|
|
Entropy
is a thermodynamic parameter that is related to the disorder of a system.
It is an extensive property, i.e. it depends upon the mass of the system.
Specific entropy is the entropy per unit mass of a system. The units of
entropy are kJ/K, and for specific entropy kJ/kg.K
Entropy
is a property of the state of a system, and the change in entropy in going
from an initial to a final state is independent of the path taken. The
entropy change will be the same for both reversible and irreversible processes
linking the two states. For a reversible process linking two states the
change in entropy DS
is given by:
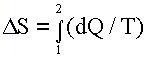 where
dQ is the path dependent heat addition. For a system that is described
by the thermodynamic probability W, the entropy may be written as: S =
k Ln W, where k is Boltzmann's constant. where
dQ is the path dependent heat addition. For a system that is described
by the thermodynamic probability W, the entropy may be written as: S =
k Ln W, where k is Boltzmann's constant. |
|
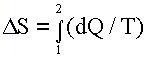 where
dQ is the path dependent heat addition. For a system that is described
by the thermodynamic probability W, the entropy may be written as: S =
k Ln W, where k is Boltzmann's constant.
where
dQ is the path dependent heat addition. For a system that is described
by the thermodynamic probability W, the entropy may be written as: S =
k Ln W, where k is Boltzmann's constant.