|
|||||||||||
|
|||||||||||
| Iron
is a metal in group VIII of the periodic table with atomic number 26, an
atomic weight of 55.85, and a density of 7.86. It has a melting point of
1539 C.
The electronic configuration of the Fe atom is (Ar)(3d)6(4s)2, and it has an atomic radius of 0.126 nm. The radius of Fe2+ is 0.076 nm, and that of Fe3+ is 0.064 nm. At
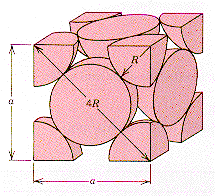
room temperature, a-Fe
has a body-centered -cubic crystal structure with a basis of a single Fe
atom. At
At room temperature, E = 208 GPa, sy = 50 MPa, UTS = 200 MPa, and a fracture strain of 0.3. |
|||||||||||
 |
|||||||||||
 |
|||||||||||
| From: Callister, "Materials Science and Engineering," Wiley (1997) | |||||||||||