|
|||||||||||
|
|||||||||||
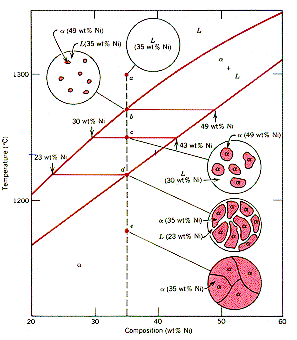
| Shown
is a partial binary phase diagram for the Copper-Nickel system with the
alloy Cu-35 wt% Ni at the vertical line. At the point, a, the alloy is
a liquid. Upon cooling it passes through the two-phase (a
+ L) zone and then
solidifies as a single phase substitutional alloy. Between (b) where
the composition line intersects the liquidus line and (d) where it intersects
the solidus line, the system exists as two phases in equilibrium.
The
Lever Rule permits the quantity of each phase present to be determined.
If C0 is the mean
composition of the alloy and Ca
and
CL are the points
at which an isotherm cuts the solidus and liquidus lines, respectively,
then the weight in the a-phase
is given by:
|
 |
||||||||||
| From:
Callister,
"Materials Science and Engineering," Wiley (1994) |
|||||||||||