|
|
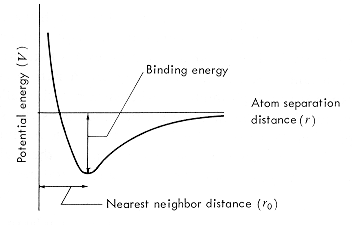
The
interatomic potential describes the interaction between a pair of atoms
or the interaction of an atom with a group of atoms in a condensed phase.
The potential must have both an attractive and a repulsive component if
binding is to occur. The form of the potential energy as a function of
lattice spacing (interatomic distance, r) in a solid is illustrated. Each
atom in the ideal crystalline solid experiences the same potential due
to the other atoms in the material.
The
binding energy of the atom in the solid is the depth of the potential well
at its minimum. The location of this minimum determines the nearest neighbor
distance, r0 , for
atoms in the solid. |
|
|
|
