|
|
When
producing metals from ores by a furnace melting process (smelting) it is
common to have some unwanted SiO2
in the ore. This material has a melting point of about 1780 C and is not
easily removed from the metal system which will have a lower melting temperature.
To remove the SiO2 economically
it is common to add a basic flux, such as CaO, to the melt. This reacts
with the SiO2 to give
a low melting point calcium silicate, CaO.SiO2,
in the reaction: SiO2
+ 2 CaO = 2CaO.SiO2.
This material has a lower density than the molten metal and will float
to the top of the melt where it can be run off removing the SiO2
contamination. This calcium silicate reaction product is known as Slag.
Incomplete
slag removal from the melt will result in calcium silicate inclusions will
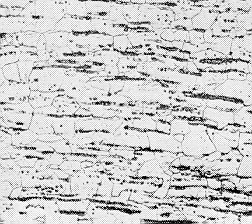
be left in the ingot, which alters its the mechanical properties. The photograph
shows a longitudinal section of a wrought iron sample. The black regions
are slag fibers that have been oriented during the manufacturing process. |
|
|
|
|
|
|
|
|