|
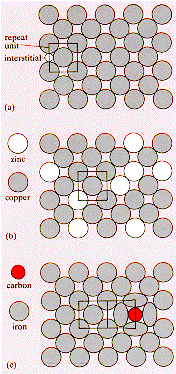
A
solid solution has atoms of a second component, the solute, dissolved in
the crystal structure of the host lattice, the solvent. The diagram illustrates
this for fcc crystal structures: (a) is the host lattice showing a {1 0
0} plane and an interstitial location on the cube edge <1 0 0 >. (b)
represents the copper-zinc substitutional alloy (a brass) where zinc atoms
are located at normal lattice sites in the copper lattice replacing the
copper atom. In (c) the fcc form of iron, Austenite, is shown with an interstitial
solid solution of carbon in its lattice. The carbon atoms locate on cube
edges and cause local lattice distortion.
Both
the copper-zinc alloy and the iron-carbon alloy have solubility limits
for the solute atoms. If this limit is passed, the solid solution separates
into two distinct phases, one of which will be of the type illustrated
and the other a compound or a second solid solution of different composition.
From:
Newey and Weaver, "Materials Principles and Practice," Butterworth (1990) |
|
|
|
|
|
|